Interpretation
Neural tumor.
Explanation
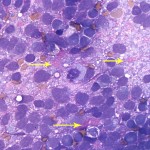
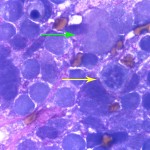
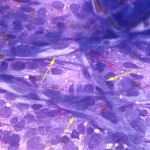
The aspirate was markedly cellular, consisting of individualized and densely packed medium to large mononuclear cells with many ruptured cells seen as bare nuclei (Figure 1). In some areas of the smear, the cells appeared more cohesive. The intact cells had round to indented to pleomorphic nuclei with finely stippled chromatin and indistinct nuclei and small amounts of deep blue granular cytoplasm. Low numbers of neoplastic cells had large nuclei with a prominent nucleolus. The cells exhibited moderate to marked anisocytosis and anisokaryosis and several mitotic figures were seen (Figure 2 and 3). The cells were embedded within a fibrillar to granular to globular bright pink extracellular matrix (Figure 2), with numerous vascular profiles (Figure 4). Low numbers of vacuolated microglia were seen, some of which had whorls or circles of pink grainy material in their cytoplasm (phagocytized myelin, presumptive). A small amount of myelin was also seen extracellularly (not shown). There was no evidence of inflammation. The results indicated a neural tumor with the primary differential diagnosis being a medium or high grade astrocytoma or primitive neuroectodermal tumor (Question 1).
Additional information
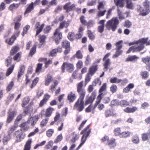
Histopathological examination of the surgical biopsy of the brain mass revealed an unencapsulated, highly cellular, infiltrative neoplasm composed of polygonal to spindle-shaped cells arranged in solid sheets and streams on scant fibrous stroma (Figure 5). The cells had indistinct margins and moderate amounts of vacuolated or slightly fibrillar amphophilic cytoplasm. The nuclei were round to oval or irregularly shaped with densely clumped chromatin. Anisocytosis and anisokaryosis were moderate to marked. Up to 7 mitotic figures were counted in 10 high power fields (400x). There were also small numbers of scattered individual necrotic cells with nuclear pyknosis. Based on these findings, a morphological diagnosis of anaplastic (medium grade) astrocytoma was made. The cells were negative on immunohistochemical staining with anti-glial fibrillary acidic protein (GFAP) and vimentin antibodies. Given the morphologic features of the neoplastic cells, a final diagnosis of an anaplastic astrocytoma that had lost GFAP expression was made.
Discussion
The incidence of intracranial neoplasms in dogs is reported to be 14.5 per 100,000 of the population at risk. Intracranial tumors are also found in 1-3% of necropsies in dogs.1,2 Over 70% of primary tumors of the central nervous system (CNS) occur in dogs over 6 years of age, unlike humans in which CNS tumors mostly affect children up to 15 years of age.2 In one study, 46% of 79 dogs with intracranial neoplasms presented with epileptic seizures.2 For tumors located in the temporal or piriform area, epilepsy may be the only clinical neurological sign, as in the present case.2
Meningiomas, gliomas (astrocytomas, oligodendrogliomas), and pituitary tumors are the most commonly recognized primary neoplasms of the CNS of dogs.1 Other primary tumors, such as ependymoma, choroid plexus tumors, embryonal tumors (primitive neuroectodermal tumors, neuroblastoma, ependymoblastoma, ectopic ectopic nephroblastoma), hematopoietic tumors (lymphoma, microgliomatosis, histiocytic sarcomas), other gliomas (glioastrocytoma, gliosarcoma, gliomatosis cerebri, spongioblastoma), and granular cell tumors can also occur.2,3,5 Primitive neuroectodermal tumors are embryonal tumors and defined as a small cell tumor arising from a progenitor cell population capable of divergent differentiation along neuronal, ependymal, glial, and possibly mesenchymal cell lines.3 Rare CNS tumors include pineal tumors, germ cell tumors, primary CNS melanoma, and chordoma (typically in ferrets but very rare in dogs).3 Primary CNS tumors are three times more common than metastatic tumors. Brain metastases can be seen with hemangiosarcoma, lymphoma, and malignant melanoma and rarely with other sarcomas, carcinomas and histiocytic tumors in dogs (Question 2).3,5
Astrocytomas account for approximately 10% of CNS tumors in dogs and occur most frequently in the cerebral hemispheres, predominantly in the temporal-piriform region, as seen in this case. However, they can also occur in other areas, including the brainstem, cerebellum, and spinal cord.2 Histological features of astrocytomas vary and they are classified into low grade (well-differentiated), medium grade (anaplastic), and high grade (also called glioblastoma multiforme) neoplasms based on morphological features of differentiation. Well-differentiated astrocytomas are slowly growing with a low mitotic rate and are further subdivided histologically into fibrillary, protoplasmic, and gemistocytic types.3 Medium grade (anaplastic) astrocytomas are pleomorphic, demonstrate nuclear atypia and more mitotic activity, but lack the vascular proliferation which is characteristic for a high-grade astrocytomas (glioblastoma). Glial Fibrillary Acidic Protein is a commonly used astrocytic marker and is typically highly expressed in low grade astrocytomas while variable expression is seen in medium or high grade astrocytomas.3
Cerebrospinal fluid analysis and aspiration or biopsy of the mass can be performed to obtain a diagnosis of a suspected brain tumor. Inflammatory pleocytosis or increased protein concentrations without a pleocytosis can be seen in some patients with brain tumors, but CSF analysis is frequently normal, as in the present case. In the absence of overtly neoplastic cells, there are no defined CSF results, including types of pleocytosis, that are specific to tumors.4,6,7 Neoplastic cells may or may not be seen in CSF. Exfoliation of neoplastic cells are not typical in meningiomas,6 but was seen in 47% of choroid plexus carcinomas in one study.8
Cytologic smears of brain tumors can be obtained by stereotaxy or open approaches. Tumors can reliably distinguished from inflammatory lesions, but a more specific diagnosis of tumor type is often not possible. This is because specific tumors can have quite variable morphologic features, which frequently overlap with other tumors. Histopathologic examination and immunostaining is usually required for a definitive diagnosis of tumor type. Table 1 summarizes reported cytologic features of several primary brain tumors in dogs.2,4,5,9
| Tumor type | Cytologic features |
| Astrocytoma | Morphological appearance varies with histological subtype; distinguishing features are hypercellularity, nuclear atypia and prominent, long, thin cytoplasmic fibrillar processes. Nuclei of neoplastic astrocytes tend to be larger and more pleomorphic than normal or reactive astrocytes. |
| Meningioma | Morphologic features are variable because they are histologically classified into at least 7 subtypes. Neoplastic meningothelial cells are often characterized by spindle-shaped cells draped around vessels and arranged in large whorling structures. A meningioma is the top differential diagnosis if an aspirate from an extramedullary brain or spinal mass reveals spindle cells. |
| Oligodendroglioma | Smears are characterized by large numbers of prominent, finely branching blood vessels with glomeruloid-like capillary tufts at low magnification. Neoplastic oligodendrocytes are relatively small, closely packed and have small amounts of granular cytoplasm with uniformly round nuclei and a small indistinct nucleolus. |
| Choroid plexus tumor | Neoplastic cells are often arranged in broad sheets of a single layer of epithelial-like cells or, less commonly, in distinctive radiating patterns similar to normal choroid plexus. The cells can consist of a regular layer of columnar epithelium with basally oriented nuclei and prominent apical cytoplasm. |
| Ependymoma | Highly branched blood vessels can be seen. The vessels are thickened and prominent due to closely attached layers of neoplastic ependymal cells, which are perpendicularly oriented to the vascular core. |
| Primitive Neuroectodermal Tumor (PNET) | Highly cellular, composed of individual or clusters of large round cells, with a moderate to high nuclear cytoplasmic ratio. Morphologic features resemble large lymphocytes (lymphoma) but they demonstrate adherence. |
| Primary CNS lymphoma | Neoplastic cells are individually dispersed round cells that are usually uniform in size. |
| Primary histiocytic sarcoma | Neoplastic cells stayed closely compacted around blood vessels. Cells are usually round to ovoid and discrete. They vary in size and have a variable amount of eosinophilic granular cytoplasm (cytoplasm is more abundant than is typical for lymphoma). Nuceli are usually eccentric and can be round to indented to pleomorphic. Bi- or multinucleated cells as well as large numbers of mixed inflammatory cells can be seen. |
Treatment for a brain tumor is aimed towards controlling secondary effects, such as increased intracranial pressure or cerebral edema or reducing tumor size through surgery, chemotherapy, radiation therapy or immunotherapy. Palliative therapy alone results in a short survival time (median, 56 days), whereas radiation therapy, alone or with surgery, can yield longer survival times (ranging from 160 to 360 days).3
Follow-up
Due to the poor prognosis, the dog was euthanized a week after the results of the surgical biopsy.
References
- LeCouteur RA, Withrow SJ. Tumors of the Nervous System. 2013. In: Withrow and Macewen’s Small Animal Clinical Oncology. Withrow SJ, Vail DM, Page R. 5th ed, pp. 659-686. Elsevier, St. Louis, MO.
- Willson DW, Dungworth DL. Tumors of Nervous System. 2002. In: Tumors of Domestic Animals. Meuten D. 4th ed, pp. 697-754. Iowa State Press.
- Koestner A, Bilzer T, Fatzer R, Schulman FY, Summers BA, Van Winkle TJ. Histological Classification of Tumors of the Nervous System of Domestic Animals. 2nd series. pp17-38, 1999. Armed Forces Institute of Patholoogy. Washington DC.
- Cook JR, Levine GJ. Cerebrospinal fluid and central nervous system cytology. 2014 In: Cowell and Tyler’s Diagnostic Cytology and Hematology of the Dog and Cat. 4th edi. 222-243. Valenciano AC, Cowell RL. Elsevier, St Louis, MO.
- Moore, PF. A review of histiocytic diseases of dogs and cats. 2013. Vet Pathol, 51:167-184.
- Dickinson PJ, Sturges BK, Kass PH, et al. Characteristics of cisternal cerebrospinal fluid associated with intracranial meningiomas in dogs: 56 cases (1985-2004). 2006. J Am Vet Med Assoc. 228:564-567.
- Bailey CS, Higgins RJ. Characteristics of cisternal cerebrospinal fluid associated with primary brain tumors in the dog: a retrospective study. J Am Vet Med Assoc. 1986. 188:414-417.
- Westworth DR, Dickinson PJ, Vernau W, et al. Choroid plexus tumors in 56 dogs. (1985-2007). J Vet Intern Med. 2008. 22:1157-1165.
- Vernau, KM, Higgins, RJ, Bollen, AW, Jimenez, et al. Primary canine and feline nervous system tumors: Intraoperative diagnosis using the smear technique. 2001. Vet Pathol. 38:47-57.
Authored by: Midori Asakawa (Clinical pathology resident), T Stokol.