Interpretation
Adrenal carcinoma and associated hyperadrenocorticism
Explanation
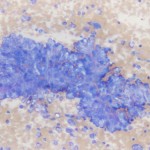
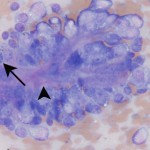
The cytology is highly cellular and contains many clusters of epithelial cells, along with a large amount of blood and low numbers of blood associated leukocytes. Low power magnification (Figure 1) reveals large sheets and cords of anaplastic epithelial cells. At higher power (Figure 2b), many cytologic criteria of malignancy are observed, including anisocytosis, anisokaryosis, increased nuclear to cytoplasmic ratios and mitotic figures (arrow). There is also a small amount of extracellular matrix weaving between the epithelial cells (arrowhead).
The findings are compatible with a carcinoma and given the location of the tumor and clinical signs, the origin is likely adrenal. Adrenal gland disease in ferrets includes hyperplasia (56%), adenoma (16%) and adenocarcinoma (26%), all of which usually result in the overproduction of sex hormones and associated hyperadrenocorticism (HAC). Most commonly the left adrenal gland is involved but there is also a high incidence of right adrenocortical disease (made more complicated by the proximity to the caudal vena cava and caudate liver lobe) and approximately 12% are bilateral.5
|
|
|
Discussion
Hyperadrenocorticism (HAC) is the second most common endocrinopathy in domestic ferrets (after insulinoma) with an estimate of 70% of the US pet ferret population affected in 2003. It has no sex prediliction and affects mainly middle-aged ferrets with a mean age of onset of 4 years old.5 The high incidence is likely due to a genetic bottleneck from a small founder population. Unlike Cushing’s disease in dogs, ferret HAC affects the zona reticularis of the adrenal gland and thus results in a hyperandrogenism marked by increases in sex hormones. While the etiology is unknown, there is a link to early sterilization and chronic stimulation of remnant undifferentiated gonadal cells in the adrenal capsule. Neutered ferrets will release GnRH from the hypothalamus, and this hormone is secreted unchecked by the negative feedback that testosterone and estrogen would provide in an intact individual. Exacerbating the cycle further, the artificially increased photoperiod experienced by most pet ferrets (>8 hours) acts to upregulate GnRH and LH secretion due to melatonin deficiency.5
Because the zona reticularis is preferentially targeted, there will be no detectable abnormalities in plasma concentration of ACTH or alpha-MSH in ferrets with HAC. However, many will still show an increased urine cortisol:creatinine ratio. This should not be interpreted as a reflection of hypercortisolemia, but rather the result of other steroid hormones cross-reacting with the assay.4 In one study, estradiol was elevated in 36% of cases, 17-hydroxyprogesterone was elevated in 60% of cases, and androstenedione was elevated in 76% of cases. 96% of ferrets with adrenocortical disease had an elevation in at least one of the aforementioned hormones and 22% showed an increase in all three.3 A ferret sex steroid serum panel is available through the University of Tennesse that includes measurement of these hormones.7 In male ferrets, an increase in one hormone is diagnostic. In female ferrets, an increase in estradiol alone must be differentiated from other possible sources including persistent ovarian remnant, but an increase in two or more is considered diagnostic.
The clinical signs, and subsequent diagnosis of ferret HAC, are largely a result of the increased sex hormones. Retrospective analysis has shown that the most common presenting complaints include progressive alopecia (>90%) with accompanying pruritis (40%), vulvar swelling (>70% of females) and cystic prostatic enlargmenent in males – which may lead to emergency urinary obstruction, stranguria, or tenesmus. Other clinical signs include mammary gland enlargement (both sexes), muscle wasting, lethargy, aggression, and increased odor.5
Usually CBC and serum biochemistry results are within normal limits with ferret adenocortical disease, but should be done to screen for other concurrent diseases such as insulinoma. On rare occasions, pancytopenia resulting from bone marrow toxicity from prolonged hyperestrogenism can be seen.5
Further information and follow-up
Unfortunately, no follow-up was available in this particular case, however, treatment options would have included both surgical and palliative medical management. As the patient was in good health, adrenalectomy (or partial debulking) would be recommended. Survival rates after surgery have been found to be 98% for 1 year and 88% for 2 years. Differences in histopathologic diagnosis (hyperplasia vs adenoma vs carcinoma), location of mass (right vs left adrenal vs. bilateral) and extent of surgery (removal vs. debulking) were not statistically significant to survivorship.6 However, two variants of adrenal carcinoma have been described in the ferret that correlate with increased malignancy and a poorer prognosis: spindle cell and myxoid variants.1,2
Medical management only antagonizes the effects of hyperadrogism, and does not help to stop tumor growth or formation. Therefore, any secondary clinical signs from mass effect or metastasis (though rare) would not be ameliorated with medical management. Medical treatment options include quarterly injections of leuprolide acetate (Lupron), a long-acting GnRH agonist, deslorelin acetate injections to stimulate LH and FSH secretion which desensitize the pituitary by downregulating GnRH receptors, and supplementation of antiogadotropic melatonin.5
References
- Newman SJ, Bergman PF, et al. 2004. Characterization of spindle cell component of ferret (Mustela putorious furo) adrenal cortical neoplasms – a correlation to clinical parameters and prognosis. Vet Comp Onco 2: 113-124.
- Peterson RA, Kiupel M, Capen CC. 2003. Adrenal cortical Carcinomas with Myxoid Differentiation in the Domestic Ferret (Mustela putorious furo). Vet Pathol 40: 136-142.
- Rosenthal KL, Peterson ME. 1996. Evaluation of plasma androgen and estrogen concentrations in ferrets with hhyperadrenocorticism. J Am Vet Med Assoc 209: 1097-1102.
- Schoemaker NJ, Mol JA, et al. 2002. Plasma concentrations of adrenocorticotrophic hormone and alpha-melanocyte-stimulating hormone in ferrets (Mustela putorious furo) with hyperadrenocorticism. Am J Vet Res 63: 1395-1399.
- Simone-Freilicher E. 2008. Adrenal gland disease in ferrets. Vet Clin Exot Anim 11: 125-137.
- Swiderski JK, Seim III HB, et al. 2008. Long-term outcome of domestic ferrets treated surgically for hyperadrenocorticism: 130 cases (1995-2004). J Am Vet Med Assoc 232: 1338-1343.
- The University of Tennessee Clinical Endocrinology Service website. http://www.vet.utk.edu/diagnostic/endocrinology/