Interpretation
Marked thrombocytosis with circulating megakaryocytes
Explanation
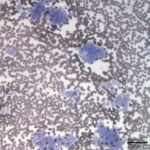
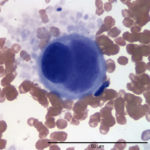
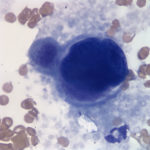
Evaluation of the blood smear revealed a marked thrombocytosis unreported by the analyzer (Question 1), demonstrating the importance of blood smear evaluation despite results falling within reference intervals. Morphologically unremarkable platelets swarm in large aggregates. While platelet aggregates are relatively common on the feathered edge of many feline smears, normal specimens do not have massive aggregates also present throughout the body of the smear (Fig. 1). This degree of thrombocytosis may cause serum pseudohyperkalemia (at least in dogs – serum chemistry was not requested on this patient) as potassium is released from the platelets during clotting. Comparison to plasma potassium levels exposes the artifact (Question 2).1 The individual large cells are megakaryocytes (Question 3), as can be discerned by their size and pink, granulated cytoplasm (Figs. 2-3), with cytoplasmic blebbing and a lobulated nucleus (Fig. 3). Although not typically visualized on blood smears, megakaryocytes circulate in the peripheral blood in low numbers in health,2 and increase with accelerated thrombopoiesis.3 Forged in the bone marrow, many circulating megakaryocytes end up in the lungs, where intravascular megakaryocytes may produce up to 50% of the total body requirement (in mice) each day.4 Although historically considered essentially spectators outside of coagulation, platelets bask in new appreciation as vital players in immunity/inflammation5 and tumor proliferation.6
|
|
Discussion
The automated enumeration of feline platelets is fraught with peril. Most automated analyzers use flow cytometry to channel individual cells in a narrow fluid stream for detection and analysis. Older electrical impedance analyzers that sort cells based merely on size (cell volume is proportional to the change in impedance) had trouble with the overlapping dimensions of the relatively large platelets and small erythrocytes of cats. Newer optical methodologies as found in our ADVIA analyzer use laser scatter to distinguish cells based not only on size (forward or low angle scatter), but also internal complexity or refractive index (high angle or side scatter) to give a 2-dimensional plot.7 However, platelets must still pass through the aperture individually (ideally, although small aggregates <60 fl in volume may be counted as one platelet with an erroneous increase in platelet volume) in order to be tabulated. Cats are near criminal platelet clumpers, harboring volatile platelets prone to activation and aggregation. Large platelet clumps fall outside the platelet counting area. Instead, they reside in a specific area near the lymphocytes, and their aggregated platelets escape enumeration. If enough incidents (typically above 1000) occur in the designated platelet clump area, the ADVIA will flag the platelet count as possibly inaccurate. For this patient, the analyzer reported 9965 platelet clumps, implying the reported platelet count of 270,000 platelets/ul did not accurately reflect the patient’s platelet status.
Individual platelet enumeration is not the only way to quantify platelets. In fact, total platelet mass may be more important for functional hemostasis than platelet number, especially in cases of macrothrombocytopenia, such as the hereditary macrothombocytopenia of Cavalier King Charles Spaniels8 or during recovery from immune-mediated thrombocytopenia.9 Platelet mass is reported as a plateletcrit (PCT), which is the percentage of the blood volume comprised of platelets, analogous to the more familiar hematocrit. The ADVIA 2120 calculates a plateletcrit (PCT) using the measured values of mean platelet volume and platelet count [PCT = (PLT x MPV) / 10,000].7 Naturally, erroneous measures of platelet number or volume or both will lead to erroneous plateletcrits. The plateletcrit reported for this patient was 0.61%. We do not have a reference interval for plateletcrits on cats, but reported values for domestic animals are typically 0.12-0.62%,8,10 with higher values in cats.10
Plateletcrit may also be measured directly. When separated by centrifugation, platelets are the least dense cellular component of blood, and perch atop the leukocytes in the buffy coat. One of the first in-house veterinary analyzers, the Idexx VetAutoreadTM, expands the buffy coat to measure plateletcrit directly, and would theoretically be useful for cats, but unfortunately, this methodology exhibits broad imprecision and inaccuracy, and clumped platelets often still generate false thrombocytopenia.11 For normal animals, the entire buffy coat is <1%, so the platelet layer is too thin for gross evaluation in a standard hematocrit tube. However, in this patient, the platelets were so markedly elevated that they were visible as a pale, translucent layer just beneath the plasma, comprising approximately 10% of the blood mass! Platelets may swell in vitro, increasing the plateletcrit, but not to this degree. From this observation, thrombocytosis was definitively diagnosed without an accurate platelet count from the analyzer.
Thrombocytosis derives from primary or secondary (reactive) etiologies.12 Spurious thrombocytosis may arise if RBC fragments or ghosts,13 lipoproteins (e.g. chylomicrons), cytoplasmic fragments from neoplastic cells, bacteria, or cryoglobulins erroneously mimic platelets during analysis.14 Primary thrombocytosis is found with myeloproliferative neoplasms (MPN) or myelodysplastic/myeloproliferative (MDS/MPN) neoplasms. These clonal proliferations have been reported rarely in the cat, and include essential thrombocythemia,15 polycythemia vera,16 and a suspected MDS/MPN with marked thrombocytosis.17 Secondary or reactive thrombocytosis is much more common, and entails thrombocytosis secondary to many causes including inflammation, neoplasia, splenectomy, iron deficiency, drug administration (e.g vinca alkaloids, corticosteroids, epinephrine) or rebound after hemorrhage or myelosuppression. Retrospective studies in cats reveal inflammation, endocrine disease (hyperthyroidism), and neoplasia as the most common associations.18,19 Severity of the increase did not correlate with clinical outcome in one study.19
These retrospective studies in cats concluded that thrombocytosis was relatively rare in cats, with an incidence of less than 5% of the hospitalized population. However, inclusion criteria for these studies required a thrombocytosis evident from hematology analyzer data. Given that aggregates may occur in 50-70% of feline specimens, artifactually reducing the count, the risk for underreporting of thrombocytosis is high. Unfortunately, platelet clumping in cats resists simple remediation. In human medicine, most platelet aggregation in EDTA tubes resolves with collection into a different anti-coagulant, commonly a citrate tube.20 This simple method is rarely effective in veterinary medicine.21,22 Collection into blood tubes individually crafted with stronger anti-aggregation components such as prostaglandin I2 analogues,23 prostaglandin E1,11 or CTAD (citrate, theophylline, adenosine and dipyridamole)24,25 reduces but does not eliminate feline platelet clumping. The added expense and inconvenience of preparing or maintaining additional blood tubes just for cats diminishes the likelihood of widespread acceptance and use, and suggests clumped cat platelets are likely to confound analyzers for the foreseeable future.
Additional Information
Additional specimens from this patient were evaluated approximately 1 week and 3 weeks after the original submission. Circulating megakaryocytes were not observed on either of these later submissions, and platelets remained severely clumped. After 1 week, the plateletcrit from a hematocrit tube decreased substantially to comprise approximately 5% of the blood. This value reflected an improving, but still marked, thrombocytosis. The analyzer reported the platelet count as within the reference interval. Two weeks later, platelets were not noticeable as a separate layer in the hematocrit tube, and appeared adequate but not increased on smear examination. Not unexpectedly, the analyzer now reported a false thrombocytopenia. Unfortunately, the clinical history remains unknown, but it seems most likely that the cat had a reactive thrombocytosis that subsequently resolved.
References
- Reimann KA, Knowlen GG and Tvedten HW. Factitious hyperkalemia in dogs with thrombocytosis. Journal of Veterinary Internal Medicine. 1989; 3: 47–52.
- Levine RF et al. Circulating megakaryocytes: Delivery of large numbers of intact, mature megakaryocytes to the lungs. Eur J Haematol. 1993; 51: 233-246.
- Breslow A, Kaufman RM, Lawsky AR. The effect of surgery on the concentration of circulating megakaryocytes and platelets. Blood. 1968; 32(3) 393-401.
- Lefrancais E. et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017 Mar 22. Doi:10.1038/nature21706. [epub ahead of print].
- Morrell CN, Aggrey AA, Chapman LM, Modjeski, KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014; 123(18), 2759-2767.
- Lin RJ, Afshar-Kharghan V, Schafer AI. Paraneoplastic thrombocytosis: the secrets of tumor self-promotion. Blood. 2014; 124(2), 184-18.
- ADVIA 2120/ 2120i Hematology Systems Operator’s Guide Computerized User Manual. 2010. Siemens Healthcare Diagnostics Inc Tarrytown, NJ
- Kelley J, Sharkey LC, Christopherson PW, Rendahl, A. Platelet count and plateletcrit in Cavalier King Charles Spaniels and Greyhounds using the Advia 120 and 2120. Veterinary Clinical Pathology. 2014; 43: 43–49.
- Schwartz D, Sharkey L, Armstrong PJ, Knudson C, Kelley J. Platelet volume and plateletcrit in dogs with presumed primary immune-mediated thrombocytopenia. J Vet Intern Med. 2014; 28: 1575–1579.
- http://www.vetmed.ucdavis.edu/vmth/local_resources/pdfs/lab_pdfs/UC_Davis_VMTH_Hematology_Reference_Intervals.pdf (retrieved 03/21017)
- Tvedten HW, Bäcklund K, Lilliehöök, IE. Reducing error in feline platelet enumeration by addition of Iloprost to blood specimens: comparison to prostaglandin E1 and EDTA. Vet Clin Pathol, 2015; 44: 179–187.
- Harrison, CN et al and British Committee for Standards in Haematology. Guideline for investigation and management of adults and children presenting with a thrombocytosis. British Journal of Haematology, 2010; 149: 352–375.
- Tvedten HW. What is your diagnosis? Discrepancy in platelet counts determined using a Sysmex XT-2000 iV hematology analyzer. Erroneous PLT-O due to RBC ghosts. Vet Clin Pathol. 2010 Sep;39(3):395-6.
- Zandecki M, Genevieve F, Gerard J, Godon A. Spurious counts and spurious results on haematology analysers : a review. Part I: platelets. International journal of laboratory hematology. 2007;29:4-20.
- Hammer AS, Couto CG, Getzy D. and Bailey, MQ. Essential thrombocythemia in a cat. Journal of Veterinary Internal Medicine. 1990. 4: 87–91. doi:10.1111/j.1939-1676.1990
- Evans LM, Caylor KB. Polycythemia vera in a cat and management with hydroxyurea. Journal of the American Animal Hospital Association 31:5, 434-438
- Weeden AL, Taylor KR, Terrell SP, Gallagher AE and Wamsley HL, Suspected myelodysplastic/myeloproliferative neoplasm in a feline leukemia virus-negative cat. Vet Clin Pathol. 2016; 45: 584–593.
- Hammar, AS. Thrombocytosis in dogs and cats. A retrospective study. Comparative Haematology International 1991. 1:181-186.
- Rizzo F, Tappin, SW, and S. Tasker. Thrombocytosis is cats: a retrospective study of 51 cases (2000-2005). Journal of feline medicine and surgery. 2007. 9: 319-325.
- Casonato A, Bertomoro A, Pontara E, et al. EDTA dependent pseudothrombocytopenia caused by antibodies against the cytoadhesive receptor of platelet gpIIB-IIIA. Journal of Clinical Pathology 1994;47:625-630.
- Wills TB, Wardrop KJ. Pseudothrombocytopenia secondary to the effects of EDTA in a dog. Journal of the American Animal Hospital Association. 2008, 44:2, 95-97.
- Stokol T and Erb, HN. A comparison of platelet parameters in EDTA- and citrate-anticoagulated blood in dogs. Veterinary Clinical Pathology. 2007. 36: 148–154.
- Riond B, Waßmuth AK, Hartnack S, Hofmann-Lehmann R, Lutz H. Effective prevention of pseudothrombocytopenia in feline blood samples with the prostaglandin I2analogue Iloprost. BMC Veterinary Research. 2015;11:183.
- Norman EJ, Barron RCJ, Nash AS, Clampitt RB. Evaluation of a citrate-based anticoagulant with platelet inhibitory activity for feline blood cell counts. Veterinary Clinical Pathology. 2001; 30: 124–132.
- Granat, F. et al. Comparison of platelet clumping and complete blood count results with Sysmex XT-2000iV in feline blood sampled on EDTA or EDTA plus CTAD (Citrate, Theophylline, Adenosine and Dipyridamole). Journal of Feline Medicine and Surgery. 2011; 13:12, 953 – 958 .
Authored by: Dr. LS Kelly