Interpretation
Marked suppurative inflammation with necrosis and fungal infection (likely Aspergillus species).
Explanation
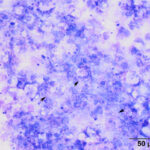
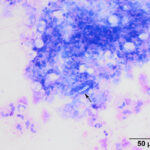
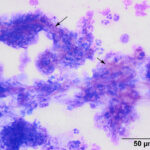
The tracheal wash was highly cellular with soft mucus, containing many enmeshed neutrophils (Figures 1-3). Neutrophils were degenerate and undergoing karyorrhexis. Non-staining septate hyphae that were parallel-sided to slightly bulbous and showing dichotomous branching (not depicted) were identified throughout the smears. Many of the hyphae did not take up stain, but were highlighted by the surrounding stained cells or background (annotated Figures 2-3 below). In some areas of the smear, neutrophils were associated with chunks and streaks of brick red to muddy purple material, compatible with necrosis (annotated Figure 4 below). Low numbers of macrophages and columnar epithelial cells and rare diplococci were seen (not shown).
The clinical pathologic abnormalities can be attributed to marked solute and fluid losses with diarrhea (relative erythrocytosis, hyponatremia), leading to hypovolemia and a type A L-lactic acidosis.
Additional tests
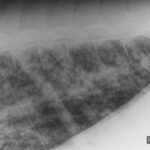
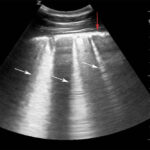
Thoracic radiography showed a diffuse nodular to interstitial pattern in the lungs (Figure 5). Thoracic ultrasonography showed diffuse pleural roughening and thickening with many ring-down artifacts (B-lines), indicating pulmonary pathology, such as edema, inflammation or neoplastic infiltrates (Figure 6). Bilateral jugular thrombosis was identified. Abdominal ultrasonography revealed fluid-filled hypomotile small intestine and a fluid-filled hypermotile large intestine, irregularities in the wall of the dorsal right colon and a fluid-filled distended stomach.
Aerobic and anaerobic blood were negative, however Aspergillus fumigatus and low numbers of Pseudomonas aeruginosa and Acinetobacter baumannii were cultured from the tracheal wash on fungal and aerobic culture, respectively. No organisms were detected on anaerobic culture of the tracheal wash. The fungus was sensitive to voriconazole but resistant to flucoconazole. The horse had an antibody titer of 1:800 against Neorickettsia risticii and was positive on polymerase chain (PCR) testing for the organism, compatible with Potomac Horse Fever, which explains the clinical signs of colitis. Fecal testing for beta-coronavirus by PCR was negative.
Follow up
A poor prognosis was given and the horse was humanely euthanized. A necropsy examination revealed a severe pyogranulomatous pneumonia with intralesional fungi, vascular necrosis and thrombosis, a fungal pyogranulomatous nephritis, a severe ulcerative lymphoplasmacytic typhlitis and lymphocytic eosinophilic colitis, with a moderate chronic lymphoplasmacytic and eosinophilic enteritis of the small intestine. There was centrilobular hepatocellular necrosis, a valvular endocarditis, and bilateral jugular thrombosis. The final diagnosis was disseminated fungal infection and chronic inflammatory bowel disease with acute Potomac Horse Fever.
Discussion
Aspergillus fungal pneumonia or equine pulmonary aspergillosis is rare in horses, with an estimated prevalence of <1%.1,2 Dual infections with other organisms, including Rhizopus and Absidia have been reported.3,4 All ages of horses can be affected, including foals.5 Other fungal organisms that can cause pneumonia include, but are not limited to, Cryptococcus, Blastomyces, Coccidioides, Histoplasma, Candida, and Pneumocystis.6 Aspergillus is usually found in the soil and plant material (decaying or alive) and horses potentially acquire infection from inhalation or ingestion of spores, although it is thought that penetration via a compromised intestinal tract with dissemination to the lungs is the most common route of infection.1,2,7,8 In this case, the route of entry was speculated to be the ulcerated cecum, with hematogenous spread to the lungs, kidney and likely heart valve. Immunosuppression due to pars pituitary intermedia dysfunction, neoplasia (e.g. acute myeloid leukemia, hemangiosarcoma) and corticosteroid therapy may also contribute to pulmonary aspergillosis.1,2,9–12
This case is unusual because the diagnosis of fungal pneumonia was made antemortem on cytologic examination of the tracheal wash. Most of the cases of aspergillus pneumonia have been diagnosed on post-mortem examination.1,2,4,5,9,11–13 In two case series of Aspergillus pneumonia in 261 and 192 horses, only 5 horses had a clinical or suspected diagnosis of pneumonia. Most of the horses in both case series presented for enterocolitis (usually acute). The authors of the 26-horse case series recommended that disseminated aspergillosis be considered as a differential diagnosis in horses not responding to antimicrobrial therapy.1 Horses with aspergillus pneumonia that do not have gastrointestinal disease may be immunosuppressed, although there are reports of the disease in horses without obvious causes of immunosuppression.13
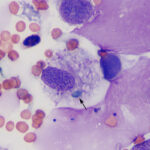
It can be difficult to conclusively diagnose fungal pneumonia in horses from cytologic examination of respiratory secretions. This is because fungal hyphae and spores can be seen in tracheal washes from healthy horses and horses with respiratory disease, with fewer organisms in bronchoalveolar lavages. In one study of 731 horses, fungal elements were observed in smears from tracheal wash samples in 79% of horses, including horses with mild to moderate asthma and non-asthmatic horses. Fungi were cultured from 55% of the horses and were mostly Penicillium and Aspergillus species.14 The presence of fungi in respiratory secretions likely represents inhalation of environmental organisms associated with feed, bedding material, or stabling. The fungi are usually dematiaceous, such as Alternaria species, with spores and fragments of hyphae seen extracellularly or associated with (and presumably attached to) macrophages (Figure 7) but fungi with features compatible with Aspergillus or Penicillium can also be seen (personal observations). Aspergillus and Penicillium produce parallel-sided, septate hyphae, which are typically blue and show dichotomous branching. They also produce 3-5 μm green cup-shaped spores. Fruiting bodies or conidiophores can help distinguish between Penicillium and Aspergillus, but are rarely seen (check out the infectious agent cytology gallery for an example of a fruiting body). The classic morphologic features may be altered within the tissue environment and Aspergillus hyphae with bulbous dilated ends have been seen in cytologic specimens (personal observations). Degrading organisms can be colorless and difficult to identify when embedded within necrotic cellular debris. Speciation on cytology is, at most, tentative, and should be confirmed by fungal culture or molecular genetic testing (e.g. 28S ribosomal DNA), which can be done on tracheal wash samples. In this case, the large numbers of fungal hyphae, necrosis, and marked inflammation with dying neutrophils indicated a pathogenic fungal infection with the fungi having features of Aspergillus. Inhaled fungi, including dematiaceous species, may be innocuous and just reflect exposure to a moldy environment, however they are believed to contribute to equine asthma. In one study, horses in which fungal elements were observed in tracheal washes had a 2.1 odds ratio of having asthma versus those without fungi.14
On histologic examination, there is a nodular to coalescing bronchopneumonia, with nodules consisting of central necrosis, surrounded by inflammatory cells (neutrophils or a mixture of neutrophils and macrophages) and fibrosis. Numerous fungal hyphae are present within the lesions and, if needed, can be highlighted by Periodic-acid Schiff and silver stains.1–5,8,12,13,15 The latter stains do outline the structure of the hyphae, allowing for tentative speciation,2–4,13 although definitive speciation is accomplished by culture (as in this case),4,8,12,13,15 immunohistochemical staining,3,4,11 or molecular genetic testing.5 Thrombi, vasculitis and intravascular fungi are commonly seen.2–5,8,13 The fungus can disseminate to the kidney, brain, heart and guttural pouch.8,13 A few horses can have chronic pneumonia.2,11 In horses with gastrointestinal disease, organisms are rarely seen in the intestinal lesions, which are usually ulcerative or necrotizing.2–5,8 Similar findings were evident in this case. Thrombosis and necrosis is not limited to Aspergillus and can be seen with other disseminated fungal infections, such as mucormycosis due to Absidia.16 A unique feature of Aspergillus infection is the formation of calcium oxalate crystals, secondary to oxalate production by the fungus. Crystal deposition in tissues is most frequently reported in birds and is rare in horses (lungs, kidney).5,17 Various species of Aspergillus have been associated with calcium oxalate crystal formation. The pathogenicity of the crystals is not clear;5,17 in one equine case, they were associated with renal nephrosis.5 Crystals can be readily missed and are best identified with polarizing light.17 The presence of calcium oxalate crystals in tissues of animals is uncommon and should trigger evaluation for underlying Aspergillus infection.
Horses with pulmonary aspergillosis have a poor prognosis due to limited availability, low efficacy, and narrow therapeutic and toxicity ratios of antifungals, emerging drug resistance, and the need for extended treatment, which is costly.6,18 There has been one report of successful treatment of a foal with voriconazole. The foal was diagnosed after surgical treatment of a suspected congenital pulmonary bulla, that was actually a cavitary pneumonia due to Aspergillus.15
Author: T Stokol.
Acknowledgment: The author thanks Dr. Amy Todd-Donato for help with the interpretation of the ultrasonographic images.
References
- Sweeney CR, Habecker PL. Pulmonary aspergillosis in horses: 29 cases (1974-1997). J Am Vet Med Assoc. 1999;214:808–11.
- Slocombe RF, Slauson DO. Invasive pulmonary aspergillosis of horses: an association with acute enteritis. Vet Pathol. 1988 Jul;25(4):277–81.
- Thirion-Delalande C, Guillot J, Jensen HE, Crespeau FL, Bernex F. Disseminated acute concomitant aspergillosis and mucormycosis in a pony. J Vet Med A Physiol Pathol Clin Med. 2005 Apr;52(3):121–4.
- Carrasco L, Tarradas MC, Gómez-Villamandos JC, Luque I, Arenas A, Méndez A. Equine pulmonary mycosis due to Aspergillus niger and Rhizopus stolonifer. J Comp Pathol. 1997 Oct;117(3):191–9.
- Hattab J, Vulcano A, D’Arezzo S, Verni F, Tiscar PG, Lanteri G, et al. Aspergillus Section Fumigati Pneumonia and Oxalate Nephrosis in a Foal. Pathogens. 2021 Aug 26;10(9):1087.
- Stewart AJ, Cuming RS. Update on fungal respiratory disease in horses. Vet Clin North Am Equine Pract. 2015 Apr;31(1):43–62.
- Hattel AL, Drake TR, Anderholm BJ, McAllister ES. Pulmonary aspergillosis associated with acute enteritis in a horse. J Am Vet Med Assoc. 1991 Sep 1;199(5):589–90.
- Pace LW, Wirth NR, Foss RR, Fales WH. Endocarditis and pulmonary aspergillosis in a horse. J Vet Diagn Invest. 1994 Oct;6(4):504–6.
- Blue J, Perdrizet J, Brown E. Pulmonary aspergillosis in a horse with myelomonocytic leukemia. J Am Vet Med Assoc. 1987 Jun 15;190:1562–4.
- Buechner-Maxwell V, Zhang C, Robertson J, Jain NC, Antczak DF, Feldman BF, et al. Intravascular leukostasis and systemic aspergillosis in a horse with subleukemic acute myelomonocytic leukemia. J Vet Intern Med. 1994 Aug;8:258–63.
- Carrasco L, Mendez A, Jensen HE. Chronic bronchopulmonary aspergillosis in a horse with Cushing’s syndrome. Mycoses. 1996 Dec;39(11–12):443–7
- Long JR, Mitchell L. Pulmonary aspergillosis in a mare. Can Vet J. 1971 Jan;12(1):16–8.
- Headley SA, de Carvalho PH, Cunha Filho LFC, Yamamura AAM, Okano W. Equine pulmonary aspergillosis with encephalitic, myocardial, and renal dissemination. Mycopathologia. 2014 Feb;177(1–2):129–35.
- Dauvillier J, Ter Woort F, van Erck-Westergren E. Fungi in respiratory samples of horses with inflammatory airway disease. J Vet Intern Med. 2019 Mar;33(2):968–75.
- Hilton H, Galuppo L, Puchalski SM, Johnson L, Robinson K, Mohr FC, et al. Successful treatment of invasive pulmonary aspergillosis in a neonatal foal. J Vet Intern Med. 2009 Apr;23(2):375–8.
- Guillot J, Collobert C, Jensen HE, Huerre M, Chermette R. Two cases of equine mucormycosis caused by Absidia corymbifera. Equine Vet J. 2000 Sep;32(5):453–6.
- Payne CL, Dark MJ, Conway JA, Farina LL. A retrospective study of the prevalence of calcium oxalate crystals in veterinary Aspergillus cases. J Vet Diagn Invest. 2017 Jan;29(1):51–8.
- Seyedmousavi S, Guillot J, Arné P, de Hoog GS, Mouton JW, Melchers WJG, et al. Aspergillus and aspergilloses in wild and domestic animals: a global health concern with parallels to human disease. Med Mycol. 2015 Nov;53(8):765–97.