Interpretation
Metastatic neoplasia (amelanotic melanoma or carcinoma) and lymphoid hyperplasia
Explanation
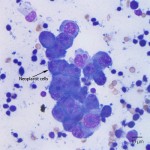
The presence of tumor cells within the lymph node indicates a metastatic tumor, with the primary differential diagnoses of amelanotic melanoma or carcinoma (Question 1). The neoplastic cells are present in cohesive groups, which would be compatible with an epithelial origin, i.e. a carcinoma. However, the cells did not display any cytologic features suggestive of squamous cell carcinoma (abundant light blue cytoplasm, occasional angular cell shape), which is the most common carcinoma in the oral cavity in dogs. Nevertheless, carcinoma should still be considered a possibility.
Given the location of the mass, the lack of squamous differentiation, and the presence of melanophages in Figure 2 (see discussion below), another important differential diagnosis to consider is an amelanotic melanoma. These can be cytologically challenging, because they may appear cohesive, spindloid, or discrete and round, so they can mimic epithelial, mesenchymal, or round cell tumors.1 In some cases, the appearance is mixed, where cells in selected areas appear epithelioid, while in other areas they appear spindloid and/or round. In one report of 70 oral melanomas in dogs, 66% were histologically described to be epithelioid, 24% were mixed, 7% were spindloid, and 3% had another appearance.2 Sometimes, careful searching will reveal low numbers of cells with a few small green-black cytoplasmic granules, but pigmented cells were not identified in this case.
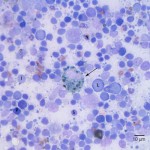
The cell indicated by the arrow in Figure 2a is a macrophage containing a large amount of coarse, large, dark green-black cytoplasmic pigment (melanin), i.e. a melanophage (Question 2). Melanin can be present either within melanocytes or phagocytized within macrophages. Melanocytes typically contain small fine cytoplasmic granulation, while melanophages contain pigment that is collected within phagocytic vacuoles so the melanin appears larger and coarser. Distinguishing whether the pigmented cells are melanocytes or melanophages is important because melanocytes should not be present within lymph nodes, so their presence would be indicative of a metastatic melanoma. Melanophages can be present in lymph nodes any time there is damage to melanocytes that releases free melanin into the area drained by the lymph node, whether those melanocytes are neoplastic (i.e. melanoma) or non-neoplastic (i.e. damage to normal pigmented skin). In this case of a white Maltese, it is less likely that there was normal pigmentation in the skin or mucosa in the area drained by the lymph node. Therefore, the presence of melanophages could be used to raise the clinical suspicion of melanoma, but would not be used to definitively diagnose the tumor type. The neoplastic cells in this sample do not have visible melanin, so if the phagocytized melanin is derived from a melanoma, it would imply that there are at least some areas of the tumor that are producing small amounts of melanin.
NOTE: The images from Figure 1 and Figure 2 came from different slides of the same lymph node. The neoplastic cells were only present in the first slide, while the melanophages were only present in the second slide. This highlights the importance of sampling multiple areas of the same site for cytologic evaluation, as features can be quite different on different cytology slides. The aspirates taken from the left mandibular lymph node (not shown) indicated only a reactive node, with no neoplastic cells identified.
The lymphoid population is mixed (question 3), with predominantly small lymphocytes and numerous intermediate to large lymphocytes and plasma cells. This is characteristic of a reactive lymph node. In this case, the lymph node reactivity is likely in response to both the tumor and the periodontal disease.
Further information and follow up
Histopathology was performed on two of the oral masses and the final diagnosis was amelanotic melanoma. The cells were highly atypical with no cytoplasmic pigment observed on histopathologic examination. One of the tumors was composed of both an epithelioid and spindloid component, while the other tumor contained more round cells. Small numbers of melanophages were present throughout. The initial presumptive diagnosis was amelanotic melanoma. Immunohistochemistry was performed, including S-100 and pancytokeratin (an epithelial cell marker used to identify a carcinoma). The tumor was regionally positive for S-100 and negative for cytokeratin. As indicated in the discussion below, S-100 is commonly positive in melanoma, although this test can be positive in other tumors, especially neural tumors. However, the appearance of the cells (epithelioid, spindled, and round) was not compatible with other S-100 positive tumors, allowing for the histopathologic diagnosis of amelanotic melanoma.
Thoracic radiographs showed no evidence of metastasis to the lungs. Treatment options were discussed with the owner, including radiation therapy and the melanoma vaccine. The owners elected supportive care only.
Discussion
Oral tumors are fairly common in the dog and account for about 6% of canine cancer. The most common malignant oral tumors in dogs are malignant melanoma, followed by squamous cell carcinoma, then fibrosarcoma. A variety of other malignant tumors can be found less commonly in the oral cavity in dogs, such as osteosarcoma, chondrosarcoma, lymphoma, and others. Other tumor types in the oral cavity in dogs that do not have metastatic potential include plasmacytomas and the epulides, although acanthomatous epulides can be very locally aggressive. Distinguishing the specific tumor type is important, as treatment and prognosis can be quite different.2,3
Confirming a suspected cytologic diagnosis of amelanotic melanoma can be difficult. The initial step is usually histopathology, but even histopathologically these tumors can mimic epithelial, mesenchymal, or round cell tumors.2,4 Immunohistochemistry to evaluate cell markers on the neoplastic cells is usually required for a definitive diagnosis. However, there is no single marker consistently found in these tumors, so sometimes a panel of several markers is needed. In a recent paper evaluating 49 amelanotic or poorly melanotic oral melanomas in dogs, the authors evaluated ten different markers by immunohistochemistry. None of the markers were positive in all tumors. The markers that were most commonly positive included MiTF (92%), PNL2 (90%), melan-A (82%), and S-100 (82%). The authors recommended that a panel of markers be employed when evaluating potential amelanotic melanomas.4 Of the markers listed above, the ones that are in most routine clinical use at this time are melan-A and S-100 (as used in this case). In this dog, it was suspected that the original mass diagnosed as a presumptive plasma cell tumor was likely the same mass that was noted on the current physical examination, because it appeared to arise from the previous biopsy site.
References
- Raskin R. Skin and subcutaneous tissue. In: Raskin R, Meyer DJ, eds. Canine and feline cytology, a color atlas and interpretation guide. 2nd ed. St. Louis, MO: Saunders Elsevier, 2010;26-76.
- Spangler WL, Kass PH. The histologic and epidemiologic bases for prognostic considerations in canine melanocytic neoplasia. Vet Pathol 2006;43(2):136-149.
- Liptak JM, Withrow SJ. Cancer of the Gastrointestinal Tract, Section A – Oral Tumors. In: Withrow SJ, Vail DM, eds. Small Animal Clinical Oncology. 4th ed. St. Louis, MO: Saunders Elsevier, 2007;455-475.
- Smedley RC, Lamoureaux J, Sledge DG, et al. Immunohistochemical diagnosis of canine oral amelanotic melanocytic neoplasms. Vet Pathol 2011;48(1):32-409.