Interpretation
Multiple pre-analytical errors
Explanation and Discussion
Non-pathologic variables that can affect laboratory results can be divided into pre-analytical, analytical, and post-analytical variables. Pre-analytical variables are those that affect the quality of the sample, such as the type of tube in which it was collected, the handling of the sample (e.g. transit time to lab), or the presence of lipemia, hemolysis, or icterus. Pre-analytical errors can cause false increases or decreases in various laboratory values. Analytical variables are those affecting the analysis itself, such as the type of assay used and the quality of the reagents. Post-analytical variables are those that affect reporting of results, such as ensuring that results are reported for the correct patient.
Three common pre-analytical problems were noted with this submission:
- A blood smear was not made from the EDTA tube when the sample was fresh (affecting CBC results).
- The serum was not separated from the cells in the red top tube (affecting chemistry results).
- There was a prolonged time for sample transit to the lab allowing changes due to sample aging (affecting both CBC and chemistry results).
Sample aging causes several artifactual changes on the CBC (Question 1). During transit, the cells in the EDTA tube will begin to absorb water from the surrounding plasma in the sample. This will lead to artifactual swelling of cells. For red blood cells, this usually results in a falsely increased MCV (which is only apparent when the MCV is above established reference intervals). Because the additional red blood cell water will dilute the hemoglobin in the cells, there may also be a falsely decreased MCHC, as there was in this case. The larger red blood cells occupy more space which can cause a falsely increased hematocrit as well. If the red blood cells take on enough water, some will rupture (as indicated by the slight hemolysis seen in the sample from this horse), causing a falsely decreased red blood cell count and hematocrit. The hemoglobin measurement in these cases should still be accurate, since there still should be the same number of hemoglobin molecules in a given volume of the patient’s whole blood. An estimated true hematocrit can be calculated by multiplying the hemoglobin by three. So this horse’s true hematocrit is likely about 12.4 x 3 or 37% (several points lower than the reported 41%).
Several studies have reported on the pre-analytical errors that can occur with automated CBC results due to sample storage.1-4 For these studies, aliquots of blood collected into EDTA were stored at 22°C and/or 4°C for up to 72 hours. Various tests including hematocrit, hemoglobin, MCV, MCHC, WBC count, and platelet count were measured at different time points to assess the effects of sample aging. Increased hematocrit, increased MCV, and decreased MCHC were noted in as little at 6-12 hours when samples were stored either at 22°C or 4°C, with the magnitude of the changes being most pronounced when stored at 22°C. Additionally, the total WBC count and platelet count were found to be decreased after 48 hours at 22°C. The only way to minimize these artifacts is to make sure the sample arrives at the lab as soon as possible, preferably within 24 hours, and to keep the sample cool (ship on ice packs, although avoid direct contact with the ice pack to prevent freezing and lysis of the red blood cells).
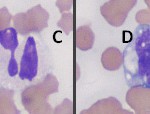
Leukocyte swelling in vitro during sample transit can alter the WBC differential and assessment of WBC morphology in several ways (Question 2). In many cases, the first change that happens is that nuclear swelling in neutrophils causes some segmented neutrophils to look like band neutrophils, making it difficult to decide if there is a true left shift in the neutrophil lineage (Figure 3, cell B). With continued cell swelling, we can often see that neutrophils rupture while lymphocytes tend to be more resistant to rupturing, making the animal appear falsely neutropenic. Eventually, all leukocytes will rupture and a differential leukocyte count cannot be performed at all. Neutrophil aging in vitro can also affect our ability to detect toxic change, as aging can cause artifactual formation of Döhle bodies and cytoplasmic vacuolation (Figure 3, cells A through C). To avoid these artifactual changes in the WBC differential and assessment of WBC morphology, a blood smear can be made from the sample when it is freshly collected. This slide need only be air-dried and submitted along with the purple top tube. This slide can also be used to better assess red blood cell morphology as it will also eliminate artifactual changes to morphology of red cells (e.g. echinocyte formation). In this case, we did not identify any of the neutrophils as band forms (since neutrophils were not obviously toxic, however a true left shift may have been missed due to this artifactual changes.
There can be multiple ways in which lack of separation of serum from cells can affect serum chemistry results (Question 3).
- It will allow cellular utilization of some serum constituents, commonly causing a falsely decreased glucose.
- There can be leakage of cellular constituents into the serum, which can be seen even when there is no visible hemolysis in the sample. This explains the falsely increased potassium (cattle and horses have high-potassium red blood cells, while most breeds of dogs and cats have low-potassium red cells, so this particular artifact is more common in samples from large animal species). At least some of the increased AST in this horse is also likely due to leakage from red blood cells, although given that there is also an increased SDH (which usually indicates hepatocellular injury), the mildly increased AST could be reflecting hepatocellular injury. Note that the GLDH (another intracellular enzyme that is specific for hepatocellular injury like SDH) is normal. We have seen falsely increased SDH (for unknown reasons) in mailed-in samples from horses and it is possible that the SDH is falsely increased in this case as well. Repeat blood sampling of the horse would be worthwhile to determine if there is true evidence of liver injury.
- If red blood cells swell to the point that there is in vitro hemolysis, artifacts in serum chemistry can develop due to several mechanisms, including the above-described leakage of cellular elements. In vitro hemolysis can also alter chemistry values through color interference (e.g. bicarbonate) or through red blood cell components interfering with the chemical reactions (e.g. CK). The mild in vitro hemolysis seen in the sample from this horse is likely not enough by itself to cause many interferences.
Some pre-analytical variables are unavoidable, such as icterus or fasting lipemia, but most are avoidable with proper sample collection and handling. Patients should ideally be fasted for 12 hours before sample collection to avoid post-prandial lipemia. For a CBC, prepare 2-3 smears from the EDTA tube when the sample is fresh (i.e. within an hour or so of collection because changes to red and white blood cells can occur within 4 hours of collection, particularly with samples that are not kept cool), and submit them concurrently with the EDTA tube. Keep the EDTA tube cool during transit (not in direct contact with ice packs!) and ship it to the lab for overnight delivery to minimize cellular swelling. For chemistry panels, centrifuge the sample promptly after collection. If collecting into a red top tube or serum separator tube, allow time for the sample to clot before centrifugation, usually 15-30 minutes. Immediately after centrifugation, transfer the serum or plasma into a clean tube with no additives. This is true even if you use a serum separator tube, as the gel in these tubes does not completely prevent cellular utilization of glucose, especially if the gel becomes dislodged during transit.
References
- Medaille C, Briend-Marchal A, Braun JP. Stability of selected hematology variables in canine blood kept at room temperature in EDTA for 24 and 48 hours. Vet Clin Pathol 2006;35(1):18-23.
- Furlanello T, Tasca S, Caldin M, et al. Artifactual changes in canine blood following storage, detected using the ADVIA 120 hematology analyzer. Vet Clin Pathol 2006;35(1):42-46.
- Bauer N, Nakagawa J, Dunker C, Failing K, Moritz A. Evaluation of the automated hematology analyzer Sysmex XT-2000iV compared to the ADVIA 2120 for its use in dogs, cats, and horses. Part II: Accuracy of leukocyte differential and reticulocyte count, impact of anticoagulant and sample aging. J Vet Diag Invest 2012;24(1):74-89.
- Warren AL, Stokol T, Hecker KG, et al. Storage-associated changes in the bovine hemogram with ADVIA 120 hematology analyzer. Comp Clin Pathol. DOI 10.1007/s00580-012-1556-9.