Interpretation
Squamous cell sarcoma; septic suppurative inflammation
Explanation
The impression smears consisted of numerous squamous epithelial cells found individually and in flat sheets (Question 1). These cells have a turquoise color cytoplasm indicating they are keratinized (Question 2a). This is abnormal, because both the normal cornea and bulbar conjunctiva are covered by nonkeratinized stratified squamous epithelial cells (Question 2b). The cells had variable nuclear to cytoplasmic ratios. Some cells had a higher nuclear to cytoplasmic ratio than normal giving them a more basilar appearance, whereas other cells (as seen in Figure 2) had more abundant cytoplasm. Many of the epithelial cells were dysplastic as evident by retained nuclei despite a mature, highly keratinized cytoplasm. Low to moderate numbers of degenerate neutrophils were also present (Question 3). Although many bacteria were present extracellularly and adhered to the squamous cells, few bacteria were phagocytized within neutrophils, confirming a bacterial infection and that they were not just contaminants (Question 4).
|
|
Case follow-up
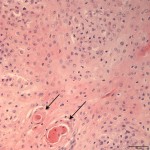
The cytologic diagnosis was confirmed on histopathology of the biopsied mass (Figure 3). Given the results, the submitting veterinarian referred the horse to the Ophthalmology Service at Kansas State University’s College of Veterinary Medicine for surgical excision of the mass. The surgery reportedly went well, and the horse was prescribed Mitomycin post-operatively to apply to the eye three times daily for a period of three weeks. Following the surgery, the horse returned to Montana and was lost to follow-up from the submitting veterinarian.
Discussion
Squamous cell carcinoma (SCC) is the most common tumor of the equine eye and adnexa.1,2 They occur most commonly in cattle, followed by horses, cats, and dogs.3 In one retrospective study covering a 17 year period, 0.6% of all horses that presented to the veterinary teaching hospital were diagnosed on histopathology with ocular or adnexal SCC.4 Out of 755 equines eyes and ocular adnexal samples submitted to the Comparative Ocular Pathology Laboratories of Wisconsin (COPLOW) over a 26 year period, 87 (11.5%) cases were diagnosed with SCC.2 Alternative differentials for ocular masses in horses include melanomas, hemagioma/hemangiosarcoma, papilloma, granulation tissue, angiosarcoma, and lymphoma.1,2,5
SCCs arise from the conjunctival epithelium of the limbus, third eyelid, or eyelid.3 From the COPLOW equine data, corneal/limbal was the most common site at 26% of cases.2 The limbus marks the transition from the avascular, nonpigmented cornea overlied by corneal epithelium, to the fibrous tissue of the sclera overlied by bulbar conjunctiva.1,3 Tumors very rarely originate from the cornea because of its limited mitotic capability.3 Therefore apparent corneal tumors are almost always extensions from tumors that arose at the limbus.3,5 In this case, the mass arose from the perilimbal bulbar conjunctiva and invaded the adjacent cornea.
The exact etiopathogenesis of SCC is unknown, but is thought to occur with increased exposure to ultraviolet radiation and a lack of protective melanin pigment.1-3 Point mutations in the tumor suppressor gene p53 consistent with ultraviolet radiation have been demonstrated in 25% (2/8) equine cutaneous SCCs.6 All breeds may be affected, but heavy draft breeds (Belgian, Suffolk, Clydesdale, Percheron, and grade draft) have been reported to be predisposed with no explained etiology.7 The current case was a Quarter Horse, which was the most common breed in one retrospective study, but may have been a factor of that hospital’s breed distribution.4 Appalossas have also been reported to be predisposed, likely because of their coat coloring.1,2 SCCs occur in a wide age range of horses, anywhere from 2 to 27.5 years old, with reported mean ages anywhere from 9.9 to 13.3 years old.2-4,7,8 Geldings were most commonly affected in one retrospective study, consistent with previous findings of a decreased incidence in sexually intact males and females.1,4 Reported clinical signs include ocular discharge, redness or discoloration, and a pink or red mass on the eye.8 The masses are sometimes described as having a “cobblestone” appearance.8 Bilateral ocular involvement is present in 15 – 20% of cases.3,4,7
Cytology of aspirates, scrapings, or impressions may be diagnostic, as in this case, however biopsy with histopathology remains the gold standard for diagnosis.5 Cytology of SCCs from a variety of anatomic sites has been described with caution to remember carcinoma with squamous differentiation and papilloma as differential diagnoses.9 In all species, SCC progresses through several premalignant stages including epidermal plaques, papillomatous-exophytic lesions, non-invasive carcinoma or carcinoma in-situ, and invasive carcinoma.2,3 Although there is a grading scheme for human squamous cell carcinomas including well-differentiated, moderately differentiated, and poorly differentiated grades, this is not used in veterinary medicine.2 However, a range of degree of differentiation is appreciated histologically in veterinary species with well-differentiated SCCs often demonstrating keratin pearl formation (as indicated by the arrows in Figure 3).3 Tumor invasion almost always occurs with a concurrent lymphoplasmacytic inflammation in these tumors, which is presumed to be a host response to tumor antigen.3 This inflammatory response is thought to be responsible for the spontaneous regression of the precancerous lesions.3
There is a suggested variant of equine ocular SCC called corneal stromal-invasive SCC, which appears clinically as a non-raised mass which grows directly deep into the corneal stroma, unlike the more common exophytic mass lesions.2 The distinguishing feature of this variant is the unusual intrastromal growth pattern with a smooth and intact anterior corneal epithelium and Descemet’s membrane in all cases.2 Interestingly, half of the cases of this variant detailed by the COPLOW group were initially misdiagnosed as chronic progressive stromal keratitis or immune mediated keratitis due to the smooth corneal surface.2 Despite their presence in the corneal stroma, the tumor islands were in contact with the limbal area in a majority of cases.2 Thirty percent of the cases in the COPLOW study were in horses with previous keratectomies, leading the authors to hypothesize that the surgery provided a stromal entrance for neoplastic cells to enter postsurgery.2 For the cases with no prior history of keratectomy, an epithelial entrance to the stroma is presumed to be present.2 Knowledge of this entity should encourage clinicians to take a deep biopsy to avoid missing the lesion.
Ocular SCCs are locally invasive with high recurrence rates and the potential for metastasis.1 Ten to 15% of equine ocular SCCs are reported to metastasize, with metastatic rates as low as 6% reported.1,3,7 Metastasis to local and cranial mediastinal lymph nodes, parotid salivary glands, and the thoracic cavity is reported.1 One study demonstrated the limbus or bulbar conjunctiva as the anatomic site with the highest rate of recurrence.4 Overall recurrence rates are anywhere from 11.9% to 46%, depending on the treatment.4,7 Patient breed, sex, and age does not appear to affect clinical outcome.7 Recurrence and clinical outcome seems to be most correlated with type of treatment and return to the hospital when recurrence was suspected.4,7 There are numerous treatment options for ocular SCC and the choice must be tailored to the patient. Factors that affect treatment decision include tumor size, location, and depth, as well as unilateral or bilateral tumors, visual status, presence or absence of metastatic disease, purpose of animal, and of course financial considerations.1 Treatment options include surgical excision, radiofrequency hyperthermia, cryotherapy, immunotherapy, radiation therapy, intralesional chemotherapy (e.g. cisplatin), carbon dioxide laser ablation, or a combination.1,4,8 If possible, complete surgical excision is the starting treatment of choice followed by some adjunctive treatment.1 In a retrospective study, recurrence rates of SCCs with or without adjuvant radiation therapy were significantly different at 11.9% and 44.1%, respectively, independent of anatomic location.4 β or γ radiation is often used as an adjunctive therapy after surgical excision/debulking or palliative treatment based on their limitations of penetration depth, which varies based on the specific radioactive isotope.1,4 Newer therapies have been researched as well. Cyclooxygenase-2 (COX-2) expression has been documented in equine ocular SCCs and immunoreactivity was associated with the mitotic index.10 COX-2 expression may be due to dysfunctional p53, as a result of ultraviolet irradiation, and a resulting lack of inhibition of COX-2.10 COX-2 expression suggests a potential therapeutic role for Piroxicam, which is a nonsteroidal anti-inflammatory drug that nonselectively inhibits COX.10
The horse in this case was treated with surgery and adjunctive Mitomycin C, a chemotherapeutic antibiotic isolated from Streptomyces caespitosus.11,12 Its active metabolite is an alkylating agent with non-cell cycle-specific antimitotic activity and generalized cytotoxicity, resulting in antineoplastic and antifibrotic activity.12 Mitomycin C has been shown to be an effective topical chemotherapeutic for a variety of ocular surface neoplasms in humans.13 It has been studied as adjunctive treatment to surgery in equine ocular SCCs with recurrence rates comparable to carbon dioxide laser ablation.12 Mitomycin C use alone has also been compared to surgery with adjunctive Mitomycin C with recurrence rates of 25% and 23%, respectively.11 Twenty-four percent of treated eyes failed to respond to Mitomycin C in that study.11 Unfortunately the horse in this case was lost to follow-up.
Acknowledgment
Thank you to Dr. Brooke Martin of Strain Equine Service LLC for the cytology and biopsy sample submission, as well as the clinical follow-up on this case.
References
- Wilkie DA. Equine ophthalmology. In: Reed SM, Bayly WM, Sellon DC. Equine Internal Medicine, 3rd ed. St. Louis, MO: Saunders Elsevier, 2010.
- Kafarnik C, Rawlings M, Dubielzig RR. Corneal stromal invasive squamous cell carcinoma: a retrospective morphological description in 10 horses. Vet Ophthal. 2009; 12 (1):6-12.
- Wilcock BP. Eye and ear. In: Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 5th ed (Volume 1). St. Louis, MO: Saunders, Elsevier, 2007.
- Mosunic CB et al. Effects of treatment with and without adjuvant radiation therapy on recurrence of ocular and adnexal squamous cell carcinoma in horses: 157 cases (1985-2002). J Am Vet Med Assoc. 2004; 225 (11):1733-1738.
- Giuliano EA, Moore CP. Eyes and ocular adnexa. In: Cowell RL, Tyler RD. Diagnostic cytology and hematology of the horse, 2nd ed. St. Louis, MO: Mosby, 2002.
- Pazzi KA, et al. Analysis of the equine tumor suppressor gene p53 in the normal horse and in eight cutaneous squamous cell carcinomas. Canc Lett. 1996; 107: 125-130.
- Schwink K. Factors influencing morbidity and outcome of equine ocular squamous cell carcinoma. Equine Vet J. 1987;19 (3):198-200.
- Rebhun WC. Treatment of advanced squamous cell carcinomas involving the equine cornea. Vet Surg. 1990; 19 (4):297-302.
- Garma-Avina A. The cytology of squamous cell carcinomas in domestic animals. J Vet Diagn Invest. 1994; 6:238-246.
- Rassnick KM, Njaa BL. Cyclooxygenase-2 immunoreactivity in equine ocular squamous-cell carcinoma. J Vet Diagn Invest. 2007; 19:436-439.
- Malalana F, Knottenbelt D, McKane S. Mitomycin C, with our without surgery, for the treatment of ocular squamous cell carcinoma in horses. Vet Rec. 2010; 167:373-376.
- Clode AB, Miller Chelsey, McMullen RJ, Gilger BC. A retrospective comparison of surgical removal and subsequent CO2 laser ablation versus topical administration of mitomycin C as therapy for equine corneolimbal squamous cell carcinoma. Vet Ophthal. 2012; 15 (4): 254-262.
- Russell HC, Chadha V, Lockington D, Kemp EG. Topical mitomycin C chemotherapy in the management of ocular surface neoplasia: a 10-year review of treatment outcomes and complications. Br J Ophthalmol. 2010; 94(10): 1316-1321.