Interpretation
Marked eosinophilic inflammation
Explanation
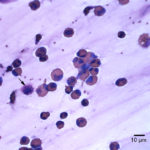
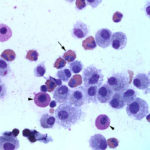
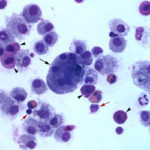
The direct smears of the fluid were highly cellular and contained stringy mucus with large numbers of eosinophils and fewer mast cell, non-degenerate neutrophils, macrophages and small lymphocytes (Figures 1a-2a). The results indicated eosinophilic inflammation (Question 1). The bronchoalveolar lavage had a normal nucleated cell count of 276 cells/uL (≤530 cells/uL1). Examination of Wright’s-stained smears of the fluid revealed 46% macrophages, some of which were erythrophagocytic, multinucleated or had phagocytized mast cell or eosinophil granules, 38% eosinophils, 10% mast cells, 2% non-degenerate neutrophils and 4% lymphocytes (Figures 3-4). The results indicated eosinophilic mastocytic inflammation with hemorrhage.
Eosinophilic inflammation to this degree is uncommon in respiratory fluids from horses. Differential diagnoses included multisystemic epitheliotropic eosinophilic disease (MEED), idiopathic chronic eosinophilic pneumonia, migrating tissue parasites (e.g. Parascaris; more common in foals), eosinophilic bronchitis associated with lung parasites (Dictyocaulus arnfieldi), lymphoma (T cell, in particular), and fungal infections (Question 2). This degree of eosinophilia is not typical of equine asthma (recurrent airway obstruction).
Additional Diagnostic Testing
Aerobic and fungal culture of the tracheal wash yielded no growth. Zinc and sugar flotation on feces revealed Oxyuris equi (pinworm) eggs. A Bauermann procedure on the feces was negative for lungworm. Polymerase chain reaction testing for Equine Herpes Virus type-5 was negative. Impression smears of the skin lesions revealed degenerate neutrophils with intracellular cocci and eosinophils. Streptococcus uberis was cultured from the skin lesions. A biopsy of the skin lesions (neck and hip) revealed serocellular crusts, loss of epidermis and extensive eosinophilic infiltrates in both biopsy sites with a secondary bacterial folliculitis. The eosinophils were found around vessels and adnexae and infiltrated between collagen bundles deep into the dermis. The histopathologic diagnosis was severe locally extensive eosinophilic inflammation with ulceration. The main differential diagnoses were allergic skin disease and MEED. Based on the pulmonary nodules on radiographic examination and eosinophilic inflammation in the tracheal wash and bronchoalveolar lavage, a diagnosis of MEED was made.
Discussion
Multisystemic eosinophilic epitheliotrophic disease is thought to be an immune-mediated disease of unknown cause involving eosinophilic infiltration of multiple organs, particularly the skin, gastrointestinal tract and lungs2. Affected horses usually present with signs of colic and diarrhea, with around 3/4 demonstrating skin lesions (alopecia, scurfiness, ulceration) that start on the limbs but become progressive. Despite the multiorgan eosinophilic infiltrates, eosinophilia is not a feature of this disease (only 6 of 46 previously reported cases had a documented eosinophilia,2,3 one of which was marked3), with most horses showing minor changes in biochemical results, typically hypoalbuminemia (23/45 horses) or increases in GGT or ALP activities (9/45 and 18/45 horses, respectively)2. The increases in cholestatic liver enzymes could be a consequence of bile duct or portal infiltration by eosinophils,2 with the high GGT activity also reflecting biliary hyperplasia. The horse in this report was hypoalbuminemic and had evidence of inflammation (neutrophilia, hyperfibrinogenemia), but no increases in liver enzymes. The inflammation could have been due to the concurrent bacterial folliculitis, although some horses with MEED do have inflammatory leukograms.2 The low albumin in this case could be secondary to an acute phase response, with downregulation of albumin production by the liver, or could reflect losses of albumin from an eosinophilic gastroenteritis (the pinworm infection would not result in gastrointestinal loss of protein), although concurrent eosinophilic inflammation in the intestine was not confirmed in this case. Not all horses with MEED exhibit diarrhea2 and the recent severe weight loss in this case would be additional support for gastrointestinal involvement.
There are no specific diagnostic tests for MEED, with the diagnosis requiring histopathologic evidence of eosinophilic infiltrates in multiple organs and lack of documentation of another disease causing eosinophilic infiltrates (e.g. fungal infection4, T cell lymphoma5). Eosinophilic infiltrates (often accompanied by lymphocytes and plasma cells) are found in the skin (around vessels and infiltrating the dermis, as in this case, with intradermal pustule formation) and multiple organs including the liver (portal and bile duct, with secondary biliary hyperplasia), pancreas, gastrointestinal system (all layers from mucosa to serosa), kidney and lungs.2 Pulmonary involvement is usually mild, but eosinophilic granulomas can form in the lungs6 and may explain the coalescing nodules seen in this case on thoracic radiography. The eosinophilic infiltrates are frequently associated with fibrosis,2 which is likely mediated through secretion of fibrogenic cytokines, such as transforming growth factor-β by eosinophils or accompanying mast cells.7 A rectal biopsy is recommended to confirm eosinophilic gastroenteritis2 but was not performed in this case. Cytologic assessment of skin lesions, pulmonary fluids or organ aspirates typically reveals eosinophilic inflammation.2,3,6 The main differential diagnoses for eosinophilic gastroenteritis are chronic idiopathic bowel disease (distinguished from MEED by intestinal-only infiltrates)8 and pythiosis.4 For pulmonary involvement, as in this case, the main differential diagnoses are parasitism (lungworm infection in adult horses, which was ruled out in this case) and idiopathic chronic eosinophilic pneumonia.9 The latter is not characterized by skin (as seen in this case) or gastrointestinal involvement (as suspected in this case) and the condition responds to immunosuppressive therapy. Some horses with the idiopathic eosinophilic pneumonia may have a mild eosinophilia (up to 2.2 x 103/uL, however only 7 cases have been reported).9
In general, the prognosis for MEED is poor, with most horses being euthanized after diagnosis.1 There is no specific treatment for the disease, however some horses have responded to treatment with immunosuppressive drugs, including hydroxyurea and dexamethasone.2
Further information and follow up
The horse was treated with antibiotics for the Streptococcus infection and anti-histamines. The horse was discharged to the care of its owner. Re-examination 1 month later showed substantial improvement of the skin lesions and pulmonary lesions and the horse had gained weight. Subsequent communication with the owner showed continued improvement with treatment. Bacteria, such as Staphylococcus, can exacerbate allergic diseases10 and eosinophilic dermatologic conditions in other species have responded to antimicrobial treatment.11 This raises the intriguing possibility that the eosinophilic skin lesions in this horse were secondary to allergies or fly bite hypersensitivity with exacerbation by the Streptococcal infection. It is unknown of the horse’s pulmonary lesions resolved with treatment, but it is possible that some horses with MEED may respond to antibiotic therapy. Streptococcus uberis is a cause of bacterial mastitis in cattle,12 but is not typically associated with skin disease, which also makes this case unusual.
References
- Couetil LL et al. Inflammatory Airway Disease of Horses – Revised Consensus Statement. Journal of Veterinary Internal Medicine (2016) 30: 503-513.
- Bosseler L et al. Equine Multisystemic Eosinophilic Epitheliotropic Disease: A Case Report and Review of Literature. New Zealand Veterinary Journal (2013) 61: 177-82.
- Latimer KS et al. Extreme Eosinophilia with Disseminated Eosinophilic Granulomatous Disease in a Horse. Veterinary Clinical Pathology (1996) 25:23-26.
- Martins TB et al. A Comparative Study of the Histopathology and Immunohistochemistry of Pythiosis in Horses, Dogs and Cattle. Journal of Comparative Pathology (2012) 146:122-131.
- La Perle KM et al. Multisystemic, Eosinophilic, Epitheliotropic Disease with Intestinal Lymphosarcoma in a Horse. Veterinary Pathology (1998) 35:144-146.
- Singh K et al. Severe Pulmonary Disease Due to Multisystemic Eosinophilic Epitheliotropic Disease in a Horse. Veterinary Pathology (2006) 43: 189-93.
- Aceves SS. Remodeling and Fibrosis in Chronic Eosinophil Inflammation. Digestive Diseases (2014) 32:15-21.
- Schumacher et al (2000) Chronic idiopathic inflammatory bowel disease in the horse. Journal of Veterinary Internal Medicine 14: 258-265.
- Bell SA et al. Idiopathic Chronic Eosinophilic Pneumonia in 7 Horses. Journal of Veterinary Internal Medicine (2008) 22: 648-53.
- Hosoki K et al. Staphylococcus Aureus Directly Activates Eosinophils via Platelet-Activating Factor Receptor. Journal of Leukocyte Biology (2012) 92:333-341.
- Koutinas AF et al. Clinical, Histopathological and Therapeutic Considerations in a Flock of Sheep with Facial Staphylococcal-associated Dermatitis. Veterinary Dermatology (2007) 18:211-216.
- Samson O et al. Use of On-farm Data to Guide Treatment and Control Mastitis caused by Streptococcus Uberis. Journal of Dairy Science (2016) 99:7690-7699.
Authored by: T Stokol