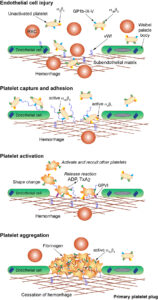
Endothelial injury: This exposes subendothelial matrix proteins, such as collagen and the high molecular weight or multimeric protein, von Willebrand factor (vWf), to circulating platelets. von Willebrand factor is normally secreted by endothelial cells constitutively into plasma and the subendothelial matrix. In plasma, it is in a tight shape which hides the binding site for adhesive receptors that are normally found on the platelet surface. Activated endothelial cells (e.g. by thrombin) release stores of ultra-high molecular weight vWf from Weibel-Palade bodies, which remain adherent to the endothelial cells or bind to extracellular matrix proteins.
Platelet adhesion: Endothelial- and matrix-bound vWf unfurls like a string under shear forces and now this multimeric protein is in the appropriate conformation to capture platelets by rapidly forming (and breaking) bonds with the platelet surface glycoprotein (GP) complex, GPIb-IX-V. This binding activates platelets and inside-out signaling changes the conformation of surface integrin receptors, including the fibrinogen receptor, αIIbβ3 (GPIIb/IIIa) and the collagen receptor, α2β1 (GPIa/IIa), moving them from an inactive (closed) to active (open) state, where they can bind ligands. These integrin-based receptors are responsible for forming slower but tighter adhesive bonds to matrix components (GPIIb/IIIa via binding to vWf-bound collagen and GPIa/IIa binding directly to collagen). GPIIb/IIIa also mediates platelet spreading on the endothelial or extracellular matrix surface and may bind platelets directly to arginine-glycine-aspartate residues (RGD) in vitronectin and fibronectin, also in the extracellular matrix. Capture by vWf is only required in vessels with high flow or shear rates, e.g. arterioles. Direct binding to collagen may be sufficient to mediate adhesion in vessels with lower shear rates on the venule side of the circulation.
Platelet activation: Once fully activated, platelets change shape, undergo phospholipid metabolism, which produces platelet agonists, such as thromboxane A2 (TxA2) and release platelet agonists, such as ADP, from dense granules. These agonists then activate and recruit unactivated circulating platelets. Thrombin, generated by simultaneously activated coagulation factors is also a potent platelet agonist, activating them by binding and cleaving protease-activated receptors (PAR) on their surfaces (PAR1, primarily). Note that GPVI, a non-integrin receptor on platelet surfaces, also binds to collagen; its main function appears to be optimizing collagen-induced platelet activation (outside-in signaling).
Platelet aggregation: As indicated above, the fibrinogen receptor (GPIIb/IIIa or αIIbβ3), once activated by inside-out signaling and in an active or open conformation, binds fibrinogen, which bridges adjacent platelets forming the platelet plug. This is sufficient to stop hemorrhage in small blood vessels (e.g. mucosal vessels) but is insufficient in larger vessels or with severe injury. In the latter situations, the platelet plug must be stabilized via fibrin formation through secondary hemostasis.
Platelet procoagulant activity: Platelets participate in secondary hemostasis (and fibrinolysis) by flipping their membranes exposing the anionic phospholipid, phosphatidylserine, which serves as a binding surface and amplification mechanism for coagulation factor complex assembly and activity. They also release phosphatidylserine-rich microparticles, which provide a large surface area on which fibrin formation proceeds. Short chain polyphosphates are also released from platelet dense granules and platelets also release stored coagulation factors, such as factor V (from alpha granules) and factor XIII and fibrinogen (from the cytoplasm). This provides high local concentrations of these factors in the vicinity of the platelets, where fibrin formation is occurring. These factors can then facilitate fibrin formation (factor V is part of the “prothrombinase” and a cofactor for activated factor X on platelet surfaces, polyphosphates promote the cofactor activity of factor V, and factor XIII crosslinks soluble fibrin).