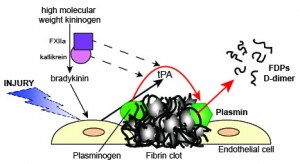
Fibrinolysis is triggered when injured endothelial cells release tissue plasminogen activator (tPA). tPA then cleaves plasminogen, which is bound to lysine residues in fibrin, to plasmin. Plasmin lyses fibrin into degradation products, including non-crosslinked fibrin degradation products (FDP) and crosslinked products of variable size (multimers), the smallest of which is D-dimer. Fibrin markedly enhances the activity of tPA, which helps localize lysis to the clot. The contact pathway factors, factor FXIIa (activated by contact) cleaves prekallikrein to kallikrein and the resulting FXIIa/prekallikrein complex converts high molecular weight kininogen into bradykinin. Bradykinin is a potent stimulus of tPA release. Both FXIIa and kallikrein can also directly activate plasminogen to plasmin but they are far weaker than tPA.
“Reproduced and modified with permission from the BSAVA Manual of Canine and Feline Clinical Pathology, 3rd edition.”