Synonyms
β-hydroxybutyrate (BHB), 3-hydroxybutyrate, 3-hydroxbutyric acid
Physiology
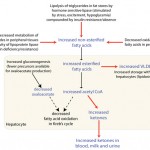
β-hydroxybutyrate along with acetone and acetoacetate, is considered a ketone. Ketones are produced from the metabolism of non-esterified fatty acids (NEFAs) and volatile fatty acids. In monogastric animals (dogs, cats), the main source of ketones is NEFAs, which are released from fat stores in the body during states of negative energy balance where fat, and noncarbohydrates (glucose) become the energy source. In ruminants, both NEFAs and volatile fatty acids produced from rumen metabolism can be used to form ketones. Propionate, butyrate and acetate are volatile fatty acids that are produced by rumen fermentation. Of these, mainly butyrate is converted to BHB in the rumen epithelium and the liver. Camelids, despite having a rumen-like forestomach, do not produce much BHB from the gastrointestinal tract.
The primary ketone produced by the liver from NEFAs is acetoacetate. This is reduced to BHB within the mitochondria and spontaneously decarboxylates to acetone. The ketones are excreted into the circulation, taken up by other tissues (e.g. skeletal muscle, mammary gland), where they are oxidized to yield energy or, in the case of the mammary gland, incorporated into milk fat. An increase in ketones in the blood is called ketosis. Since ketones are acids, increased concentrations can result in a primary metabolic acidosis when values are high enough to overcome normal body buffers (primarily bicarbonate, concentrations of which decrease in blood). This is called ketoacidosis but it is not always present in states of ketosis. Ketones are freely filtered by the glomerulus and, since renal absorptive thresholds are low, they are readily found in the urine during ketosis.
Methodology
A variety of assays are currently available for measuring BHB levels in bodily fluids: spectrophotometric quantitative assays, nitroprusside method (e.g. Ketostix, Acetest), other quantitative or semiquantitative methods (e.g. Ketolac BHB strip). Spectrophotometric assays based on the catalytic activity of BHB dehydrogenase are generally used to quantify BHB concentration, e.g. Randox assay, and is the method used at the Clinical Pathology Laboratory of the Animal Healthy Diagnostic Center at Cornell University.
Reaction type
Kinetic
Procedure
In this reaction, the enzyme 3-Hydroxybutyrate dehydrogenase catalyzes the oxidation of D-3-hydroxybutyrate to acetoacetate. This oxidative process results in the concomitant reduction of NAD+ to NADH. The amount of NADH generated is measured photometrically, at 340 nm, and is directly proportional to the concentration of D-3-hydroxybutyrate in sample (reported in mg/dL).
The reaction is shown below:
D-3 hydroxybutyrate + NAD+ 3-hydroxybutyrate dehydrogenase > Acetoacetate + H+ + NADH
Units of measurement
The concentration of BHB is measured in mg/dL (conventional units) or mmol/L (SI units). The conversion formula is shown below:
mg/dL x 0.096 = mmol/L
Sample considerations
Sample type
Serum and plasma
Anticoagulant
Heparin or EDTA
Stability
BHB values are stable for 72 hours at 4°C or 24°C in separated serum, EDTA plasma or heparin plasma from bovine blood. BHB values are stable in all three samples kept as whole blood for 24 hours in dairy cattle. Bovine BHB is also stable frozen for 1 month at -70°C (Stokol and Nydam 2005)
Recommendations for sample collection in transition dairy cows
- Collection tube: A red top tube is recommended for sample collection. EDTA- (purple top) and heparin- (green top) anticoagulant tubes can be used as well. Separate serum or plasma from cells ASAP after collection.
- Pooled samples: Pooling of individual samples from cows to assess energy status of a herd is NOT recommended. Studies at Cornell University have shown that results from pooled samples are insensitive when using prepartum or postpartum NEFA for the detection of excessive negative energy balance in transition dairy cows.
- Time of collection: Collect samples from cows that are 3-14 days in milk. Also sample cows as they are coming into the feeding stalls.
- Number of cows: A minimum of 12 animals per herd should be sampled for herd level testing. This can be a mixture of heifers and >2 parity cows.
Interferences
- Lipemia, hemolysis, icterus: None of these interferents affect the BHB concentration substantially with the methods used by Cornell University.
Test interpretation
Low values are not clinically relevant.
Measurement of BHB values may be useful in evaluating rumen development in weaned calves. With weaning and the shift from liquid to solid feed, the rumen epithelium changes to facilitate increased absorption of volatile fatty acids, the main source of energy for cattle and product of rumen fermentation by anaerobic bacteria. A study of 20 calves over weaning showed that BHB concentrations of 100 μmol/L (1.0 mg/dL) support adequate rumen maturation and feed intake from starter feed for weaning, when samples are collected 2 hours after eating the ration (Deelen et al 2016). However, these studies have just started and more data is needed to determine how useful BHB truly is for this purpose.
Increased concentration (ketonemia)
Increased BHB concentrations in blood indicates stimulation of lipolysis (all species) or excess absorption of butyrate (ruminants) from feeding spoiled silage (also called alimentary ketosis). Lipolysis is stimulated by any condition leading to negative energy balance (starvation/anorexia, late pregnancy or lactation, insulin lack/inhibition) and exercise (dogs, horses). In late pregnancy, negative energy balance may be compounded by a state of insulin resistance (due to progesterone). This promotes ketogenesis by causing a shift in energy metabolism from glucose to fat, decreasing conversion of dietary or endogenous triglycerides to stored fat, and by decreasing fatty acid oxidation in hepatocyte mitochondria (thus more acetyl CoA is available to form ketones (see image above). Note that horses have poorly developed ketogenic pathways, so ketosis is rare in this species.
- Bovine ketosis: Ketosis primarily occurs in postcalving dairy cows, who are undergoing extensive lipolysis due to a combination of decreased dry matter intake and increased energy demands (from the fetus and milk production). Many transition dairy cows are also thought to be insulin resistant, which favors lipolysis and ketogenesis. VLDL transportation from the liver is inefficient in ruminants, which contributes to hepatic lipidosis in affected animals. Ketosis can be initiated by underlying diseases, which cause decreased dry matter intake and subsequent negative energy balance. Ketosis can also be subclinical or clinical. Note that any cow (not only transition cows) can develop alimentary ketosis (excess rumen butyrate production)
- Subclinical ketosis: Postcalving dairy cows with increased BHB, but no clinical signs of ketosis (off feed, decreased milk yield), are considered to be in a state of subclinical ketosis. Associations have been made between subclinical ketosis and an increased incidence of inflammatory (metritis, mastitis) and metabolic (displaced abomasum, clinical ketosis) diseases postcalving. Subclinical ketosis is also thought to reflect underlying hepatic lipidosis. Dairy practitioners have begun to monitor dairy herds for subclinical ketosis (which is an indirect indicator of excess negative energy balance) by testing for BHBs in the postcalving period, either alone or in combination with other tests (urea, albumin, NEFA, AST) as part of transition cow energy metabolite assessment. Results of these tests can be interpreted at the individual cow level (i.e. a BHB value above a certain cut-off indicates subclinical or clinical ketosis) or at the herd level (i.e. the proportion of tested cows that have BHB values over a certain cut-off value). The following interpretation guidelines are based on studies done at Cornell University and are valid for samples collected from ‘at risk’ total mixed ration (TMR)-fed cows between 3-14 days post-calving (Ospina et al., 2013).
- Cow level testing: Post-calving BHB > 10 mg/dL is associated with a significant risk of post-calving metabolic or infectious diseases (displaced abomasum, clinical ketosis and metritis), decreased milk yield and decreased reproductive performance in individual TMR-fed Holstein cows.
- Herd level testing: At the herd-level, there is a significantly increased risk of these post-calving diseases, decreased milk production or decreased reproductive performance if >10% of tested post-calving cows have BHB values > 10 mg/dL. Note, that as indicated above, pooling samples from individual cows is not recommended for herd-level test.
- Clinical ketosis: Clinical ketosis typically occurs in cows during early lactation (usually the first 2-4 weeks) and is most frequently seen in dairy cows (due to demands of the fetus and lactation, since they are bred to be high milk producers). This is also called lactation or spontaneous ketosis and is a consequence of excess negative energy balance due to stresses of calving and lactation. Occasionally, dairy cows in late lactation can also develop clinical ketosis (pregnancy ketosis) due to negative energy balance. Affected cows are dull, inappetant, lose weight and have decreased milk yield. Cows with clinical ketosis in dairy herds fed concentrate rations are frequently concurrently hypoglycemic. This worsens the state of negative energy balance Blood, urine and milk BHB values are often quite high. Blood BHB values >27 mg/dL are considered compatible with clinical ketosis. Cows with underlying hepatic lipidosis may have concurrent increases in liver leakage enzymes (AST, SDH, GLDH) or cholestatic enzymes (GGT, ALP), although these changes are not specific for this disorder and may be normal in affected animals. Cholesterol concentrations can be decreased in cows with lipidosis and a NEFA to cholesterol ratio >0.2 (SI units) has a higher odds ratio of lipidosis and was more sensitive than AST activity (63% versus 33% at an AST activity cut-off of 120 U/L) in one study in dairy cattle (Mostafavi et al 2013).
- Beef cows can also develop ketosis, especially in the last 2 months of pregnancy, when carrying twins.
- Alimentary ketosis: Alimentary ketosis results from feeding cattle spoiled silage with excess butyric acid. A spontaneous ketosis also occurs in cows in peak lactation despite abundant, good-quality feed. The animals are not acidotic and often recover spontaneously (despite decreased milk production).
- Subclinical ketosis: Postcalving dairy cows with increased BHB, but no clinical signs of ketosis (off feed, decreased milk yield), are considered to be in a state of subclinical ketosis. Associations have been made between subclinical ketosis and an increased incidence of inflammatory (metritis, mastitis) and metabolic (displaced abomasum, clinical ketosis) diseases postcalving. Subclinical ketosis is also thought to reflect underlying hepatic lipidosis. Dairy practitioners have begun to monitor dairy herds for subclinical ketosis (which is an indirect indicator of excess negative energy balance) by testing for BHBs in the postcalving period, either alone or in combination with other tests (urea, albumin, NEFA, AST) as part of transition cow energy metabolite assessment. Results of these tests can be interpreted at the individual cow level (i.e. a BHB value above a certain cut-off indicates subclinical or clinical ketosis) or at the herd level (i.e. the proportion of tested cows that have BHB values over a certain cut-off value). The following interpretation guidelines are based on studies done at Cornell University and are valid for samples collected from ‘at risk’ total mixed ration (TMR)-fed cows between 3-14 days post-calving (Ospina et al., 2013).
- Pregnancy toxemia in small ruminants: Clinical ketosis due to excess energy demands from the fetus (particulary with twins) also occurs in sheep and goats. Since, these animals are not farmed intensively and are not selected for high milk production, subclinical ketosis is less of an issue, in terms of herd management, in these species.
- Clinical ketosis in camelids: Clinical ketosis occurs in states of negative energy balance (stress, anorexia, pregnancy, lactation) and as a complication of hepatic lipidosis in llamas and alpacas. Both males and females can develop hepatic lipidosis and ketosis; females are often in late pregnancy (called pregnancy toxemia) or early lactation. The syndrome of hepatic lipidosis develops when dietary needs are insufficient or feed intake is inadequate to meet energy demands (which are naturally increased in pregnant or lactating females). Hepatic lipidosis mostly occurs in older animals, with weight loss and anorexia being the most frequently observed clinical signs. Clinical pathology testing demonstrates increases in liver leakage (AST, SDH, GLDH) and cholestatic (GGT, ALP) enzymes. Some animals are icteric (due to cholestasis) and increased bile acids are seen due to cholestasis and decreased hepatic function. High NEFA concentrations reflect negative energy balance, as do high triglycerides (and is associated with visible lipemia in some animals). In one study, none of the affected camelids were hypoglycemic, but several were hyperglycemic (suggesting underlying insulin resistance).
- Diabetic ketoacidosis in small animals: Clinical ketosis is seen primarily in small animals as a consequence of diabetes mellitus. Lack of insulin or insulin resistance creates a state of negative energy balance due to decreased ability of cells to take up and use glucose. This is compounded by decreased metabolism of triglycerides (decreased activity of lipoprotein lipase), unopposed stimulation of hormone sensitive lipase (stimulates lipolysis), and stimulation of gluconeogenesis (which decreases oxaloacetate in hepatocyte mitochondria, facilitating ketogenesis). The concentrations of lipolytic hormones (glucagon, epinephrine norepinephrine, cortisol and growth hormone) are often increased in diabetic animals. Ketosis is thought to develop in diabetic animals due to additional hormonal and metabolic stresses imposed by underlying diseases (infection, pancreatitis, renal insufficiency). Affected animals usually have a high anion gap metabolic acidosis (from accumulation of ketones) and ketonuria. BHB concentrations (which are typically very low in health) are markedly increased. Rarely, dogs in lactation can also suffer from ketosis.
