Interpretation
Marked hemolytic anemia with intra-erythrocytic organisms compatible with Theileria species and a concurrent lymphocytosis suspected to be secondary to bovine leukemia virus (BLV) infection (persistent lymphocytosis)
Explanation
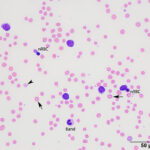
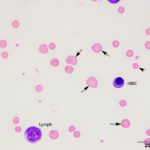
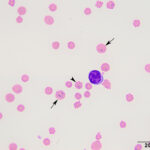
The anemia was regenerative, based on moderate polychromasia and moderate to many macrocytes in the blood smear. The macrocytes and polychromatophils explained the high MCV, high RDW (the smear equivalent of anisocytosis) and low MCHC (the latter due to the polychromasia) on the numerical hemogram results. Basophilic stippled red blood cells (RBCs), Howell-Jolly bodies, and nucleated RBCs (nRBCs) were seen in the blood smear and can be present in blood of ruminants with a regenerative anemia, although concurrent bone marrow injury from hypoxia or thrombosis could be responsible for the increased nRBCs. There were also low numbers of acanthocytes, keratocytes, and schistocytes, supporting fragmentation injury (Figures 1-2). With the concurrent mild thrombocytopenia and high MPV (supporting increased platelet production), the fragments suggested concurrent disseminated intravascular coagulation. A few putative smaller round RBCs were identified (Figure 2); these could be spherocytes, which were attributed to fragmentation, but also could indicate a concurrent immune-mediated hemolytic process. Many RBCs contain 1-4 rod-, coccoid-, comma-, signet-ring-, or racquet-shaped piroplasms (1 µm x 2-4 µm), compatible with Theileria due to the presence of long tails (Figures 1-3). Infection with Babesia species was the main differential diagnosis for the organisms. The anemia was attributed to parasite-induced extravascular hemolysis with a likely minor concurrent intravascular component, based on the mild hemolysis evident on plasma appearance in the EDTA and heparin tubes (56 hemolytic index) (Questions 1 and 2). Increased production of unconjugated bilirubin from the hemolytic anemia would explain the high total and indirect bilirubin concentrations, although anorexia may be contributing to these changes. The high iron and percentage saturation can also be attributed to the hemolytic anemia (due to increased iron turnover). The lymphocytes were predominantly small to intermediate and had clumped chromatin with small amounts of blue cytoplasm (Figures 1-3). Neutrophils were not toxic. The neutropenia with a mild left shift supported acute inflammation, which was likely due to ischemic necrosis of tissues, such as the liver, based on the changes in the biochemical panel. The lymphocytosis could be secondary to antigenic stimulation from the organism or a concurrent persistent lymphocytosis due to BLV infection and thus prompted BLV testing (Question 3, see additional tests). The latter was favored as a lymphocytosis to this degree has not been reported in cows with piroplasmosis. It is unclear how the mild diarrhea was related to the protozoal infection, however the animal could have had a concurrent underlying gastrointestinal disorder.
The acidemia on the blood gas analysis on admission and the high anion gap acidosis (low bicarbonate concentration, high anion gap) on the chemistry panel (indicating a primary titration metabolic acidosis) was attributed to an L-lactate acidosis from presumptive shock and anemia. The marked increases in SDH and GLDH activities indicated hepatocellular injury, supported by the post-mortem findings (see below). The hepatic necrosis could also partly explain the hyperferremia and increased percentage saturation of transferrin. The high AST activity can be attributed to muscle (potentially to myocarditis or skeletal muscle injury) and liver injury. The mild hypoglycemia may be due to decreased hepatic gluconeogenesis (cause unclear). The low globulin concentration (with normal albumin concentration) is difficult to explain, given the concurrent clinical dehydration, but this result persisted in a second chemistry panel done 2 days later. The low globulin concentration could be explained by a falsely increased albumin concentration due to binding of inflammatory proteins to the bromcresol green dye in heparinized plasma1 (the globulin concentration is calculated from the total protein minus the albumin concentration). Concurrent immunosuppression cannot be ruled out. The moderate hypomagnesemia could be due to gastrointestinal losses, given the history of diarrhea, but translocation intracellularly cannot be ruled out (e.g. due to epinephrine).
Additional tests
An ELISA test for BLV antigen was positive, whereas one for Anaplasma marginale was negative. Flow cytometric analysis was done on the peripheral blood in an attempt to phenotype the lymphocytes, with a limited antibody set known to cross-react with bovine leukocytes. The lymphocytes expressed CD21 (38%), MHCII (85%) and MHCI (96%), supporting an expansion of B cells as documented in other cases of bovine leukemia virus-induced persistent lymphocytosis.2 They were negative for neutrophil/monocyte markers (CD14, CD172a, CD163). Antibodies against T cell markers for flow cytometry were not available. Blood was also submitted for 18s ribosomal RNA gene sequencing of the parasite. The obtained sequence had high homology to Theileria (T.) orientalis genotype buffeli and T. sergenti. Based on the geographic distributions of these two species (North America for T. orientalis versus Japan for T. sergenti), it was deemed most likely that the organism was T. orientalis genotype Buffeli. The concentration of cardiac troponin-I was measured and was increased at 6.9 ng/mL (normal, 0 ng/mL).
Treatment and follow up
The cow was given a transfusion of 6 liters of bovine whole blood on presentation to treat the anemia and shock. Antibiotics, glucose, vitamin B, flunixin, and calcium were also given as supportive care. Urine collected during the transfusion revealed a positive reaction on the dipstick for heme and bilirubin (the latter result was not confirmed with an ictotest). The heme reaction could be due to hematuria or hemoglobinuria, with the latter suspected based on the piroplasmosis and hemolytic anemia. Bilirubinuria, if real, would support underlying cholestasis (as reflected in the post-mortem findings) that had not yet resulted in increased direct bilirubin concentrations in blood. A second blood transfusion was given and imidocarb diproprionate therapy was initiated, but the cow did not clinically improve. Repeat hemograms on days 2 and 3 of hospitalization showed persistent anemia and lymphocytosis and worsening neutropenia with a left shift, supporting an increasingly severe acute inflammatory response. A repeat biochemical profile on day 2 showed decreased activities of liver enzyme and CK activities but a higher indirect bilirubin concentration with mild increases in direct bilirubin, supporting cholestasis. The acidosis had resolved. On day 6 of hospitalization, the cow was severely tachycardic, lethargic, anorexic, and anxious, and euthanasia was elected.
The post-mortem examination revealed numerous petechial hemorrhages on the skin and tracheal mucosa. Subcutaneous and visceral adipose tissues were diffusely icteric. Peripheral lymph nodes were prominent and there was diffuse hepatomegaly and splenomegaly and an enlarged gall bladder. The spleen also contained a well-demarcated, unencapsulated, round nodule. There were numerous abomasal ulcers. There was bilateral renomegaly, with dark green staining at the corticomedullary junction, which was attributed to hemoglobinuria. Serosanguinous fluid and fibrin strands were present in the pericardium and fibrin strands were overlying the pleural surfaces. An approximately 150-180 day old fetus was present in the uterus.
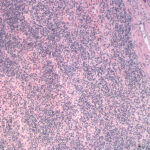
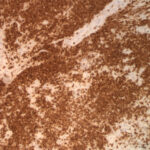
Histopathologic examination of hematoxylin-&-eosin-stained slides revealed that the lymph nodes were effaced by a monomorphic population of small lymphocytes (Figure 4). Small lymphocytes with fewer intermediate and large lymphocytes also diffusely expanded the red and white pulp of the spleen (Figure 5) and were found within the intestine, lung and hepatic sinusoids. In the intestine, the lymphocytes were found diffusely in the lamina propria and extended into the underlying submucosa and crypt epithelium. The spleen also contained moderate numbers of hematopoietic precursors and hemosiderin-laden macrophages. Within the liver, there were diffuse areas of centrilobular and midzonal hepatocellular necrosis with adjacent sinusoids containing fibrin. Hydropic degeneration was evident in hepatocytes at the periphery of the necrotic areas, with the remaining hepatocytes containing lipid. Moderate numbers of bile casts were seen, indicating concurrent cholestasis. In the heart, there was a severe, multifocal to coalescing, granulomatous to lymphoplasmacytic, fibrosing myocarditis, explaining the increased concentration of cardiac troponin-I. Immunohistochemical staining of the lymph node, spleen (Figure 6), and intestine showed strong membranous staining for CD3 in the small lymphocytes, which were negative for the B cell marker, Pax-5 (rare aggregates of Pax-5 positive B cells served as an internal control). The kidney was not sectioned for histopathologic examination. The final histologic diagnosis was a diffuse T cell lymphoma, myocarditis, renal hemoglobinuric nephropathy and hepatic necrosis and lipidosis with cholestasis.
Discussion
Theileria and Babesia are tick-transmitted Apicomplexan protozoal erythroparasites, which infect many species, including ruminants.3 These organisms are uncommon causes of hemolytic anemia in cattle in the United States and are reportable diseases. There are numerous Theileria species that infect ruminants with varying geographic ranges. The two species causing the most severe disease are T. parva and T. annulata. T. parva is responsible for the rapidly fatal disease, known as East Coast Fever, in Africa whereas T. annulata is the agent of tropical theileriosis in Africa, Asia and Europe.4,5 There are 11 genotypic variants of T. orientalis, which vary in their pathogenicity. The most well known genotypes are the first three – Chitose, Ikeda, and Buffeli. Of these genotypes, Ikeda and Chitose (particularly Ikeda) are considered more pathogenic than Buffeli and can cause outbreaks of disease, particularly in beef, but also in dairy, cattle. Infections with all three genotypes can be subclinical and affected animals are typically lifelong in carriers.3,6,7 The genotype Buffeli has been identified as a cause of anemia in cattle in the United States.8,9 T. orientalis was first reported in Kansas and Texas,3 but cases have been identified as far north as Michigan,9 likely due to widespread eastern and central distribution of the vector, the Asian longhorned tick (Haemophysalis longicornis).3,6 In one epidemiologic survey of cattle markets in Virginia, the most prevalent genotype was Ikeda.6
Following a blood meal by a tick, the organisms are released from infected RBCs into the intestine of the tick, giving rise to micro- or macrogametes, which then undergo sexual reproduction (syngamy) to yield diploid zygotes. The zygotes invade the intestinal epithelial cells in the tick and undergo further differentiation and division to yield kinetes. The kinetes enters the hemolymph and undergo nuclear division to form multinucleate sporoblasts in the salivary gland. The sporoblasts then produce uninucleate sporozoites, which are transmitted to cattle during tick feeding. Sporozoites infect lymphocytes, dendritic cells or macrophages within the animal and undergo asexual replication to produce multinucleate schizonts. After schizonts form, the parasite undergoes further nuclear division and differentiation to form single nucleated merozoites. Zoites are released from the leukocytes and invade erythrocytes, yielding a parasitemia.3 Invasion of leukocytes and RBCs can be of pathologic consequence, resulting in lymphocyte proliferation or destruction and anemia, respectively, to variable degrees that depend on the species of Theileria.10-11
The diagnosis of clinical piroplasmosis was made in this case by examination of a blood smear and identification of the organism within erythrocytes. A parasitemia occurs within 10-35 days after experimental inoculation of calves with T. sergenti and T. orientalis genotype Buffeli.8,10 Theileria merozoites are quite pleomorphic appearing as cocci, rod, comma, piriform, signet-ring, tetrad, or racquet-shaped forms in red blood cells.8 The main differential diagnosis for the organism is small Babesia, such as Babesia bovis. Distinguishing between these two organisms can be done with PCR or serologic testing, such as ELISA, although genotyping for different T. orientalis organisms would require sequencing of parasitic genes. Theileria orientalis induces a hemolytic anemia, which can be regenerative, and appears to be primarily due to extravascular hemolysis,7-9 although there can be an intravascular component.9,12 The anemia in some infected cattle may be non-regenerative.7 The cause of hemolysis is multifactorial and has been attributed to protozoal proteases, oxidative injury and eryptosis.8,13 An immune-mediated component may be present and could explain the putative spherocytes observed in the blood smear of this case.14 Spherocytes can also be attributed to fragmentation injury, potentially secondary to DIC.8 Other Theileria sp, such as T. parva, can cause a pancytopenia, with the non-regenerative anemia being attributed to parasite infection of erythroblasts (rubricytes, prorubricytes).11 The leukograms of affected animals may show a mild left shift, supporting inflammation.7 Biochemical changes are related to hemolysis, such as increased total bilirubin concentrations, and hypoxic injury to the liver, e.g. high glutamate dehydrogenase activity. The activity of GGT can also be high in infected cattle, which may reflect concurrent cholestasis.7,12 Clinical signs in infected cattle are referable to the anemia and include pallor, decreased milk production, lethargy, jaundice, and dyspnea. Abortions have also been seen in herd outbreaks.12,15
Experimental and naturally occurring infections with Theileria species is associated with a mild lymphocytosis in calves and adult cattle, usually <10,000/uL.7,10 The degree of lymphocytosis in the case of this report and other cases with dual BLV and Theileria infections8,9 were higher than 10,000/uL and were attributed to the BLV infection. However, antigenic stimulation secondary to the parasitic infection could have contributed to the lymphocytosis.
An unusual finding in this case was the presence of a concurrent small cell T cell lymphoma. Lymphomas associated with BLV infection are typically of B cell origin16 and the BLV infection is unlikely to be responsible for the T cell lymphoma. Transformation and uncontrolled proliferation with clonal expansion of schizont-containing leukocytes has been reported with T. parva and T. annulata but not Theileria orientalis infections, since the latter organism primarily proliferates in erythrocytes.17 Thus, we think it is unlikely the theilerial infection was oncopathogenic. Interestingly, many of the lymphocytes in the case herein were negative for B and T cell markers on flow cytometric testing. We cannot rule out that these negative cells represented a leukemic phase of the T cell lymphoma.
Authors: A Newman, E Behling-Kelly, H Priest, E Olson, H Daverio. This case was presented during the mystery slide session at the 2014 Mystery Slide session of the American Society of Veterinary Clinical Pathology and American College of Veterinary Pathologists annual meeting. The case was modified for this challenge by T. Stokol. We thank Dr. Elisha Frye for additional information and discussion regarding Theileria cases.
References
- Stokol T, Tarrant JM, Scarlett JM. Overestimation of canine albumin concentration with the bromcresol green method in heparinized plasma samples. Vet Clin Pathol 2001;30:170-176.
- Esteban EN, Thorn RM, Ferrer JF. Characterization of the blood lymphocyte population in cattle infected with the bovine leukemia virus. Cancer Res. 1985 Jul;45(7):3225–30.
- Almazán C, Scimeca RC, Reichard MV, Mosqueda J. Babesiosis and Theileriosis in North America. Pathogens. 2022 Jan 27;11(2):168
- Bishop RP, Odongo D, Ahmed J, Mwamuye M, Fry LM, Knowles DP, et al. A review of recent research on Theileria parva: Implications for the infection and treatment vaccination method for control of East Coast fever. Transbound Emerg Dis. 2020 Mar;67 Suppl 1:56–67
- Gharbi M, Darghouth MA, Elati K, Al-Hosary AAT, Ayadi O, Salih DA, et al. Current status of tropical theileriosis in Northern Africa: A review of recent epidemiological investigations and implications for control. Transbound Emerg Dis. 2020 Mar;67 Suppl 1:8–25
- Telionis A, Lahmers K, Todd M, Carbonello A, Broaddus CC, Bissett CJ, et al. Distribution of Theileria orientalis in Virginia Market Cattle, 2018-2020. Pathogens. 2022 Nov 15;11(11):1353.
- Lawrence KE, Forsyth SF, Vaatstra BL, McFadden A, Pulford DJ, Govindaraju K, et al. Clinical haematology and biochemistry profiles of cattle naturally infected with Theileria orientalis Ikeda type in New Zealand. N Z Vet J. 2018 Jan;66(1):21–9
- Stockham SL, et al. Theileriosis in a Missouri beef herd caused by Theileria buffeli: case report, herd investigation, ultrastructure, phylogenetic analysis, and experimental transmission. Vet Pathol 2000;37: 11-21.
- Cossio-Bayugar R, Pillars R, Schlater J, Holman PJ. Theileria buffeli infection of a Michigan cow confirmed by small subunit ribosomal RNA gene analysis. Vet Parasit 2002;105: 105-110.
- Shimizu S, et al. Clinico-hematological observation of calves experimentally infected with Theileria sergenti. Jpn J Vet Sci 1990;52(6): 1337-1339.
- Mbassa GK, Balemba O, Maselle RM, Mwaga NV. Severe anaemia due to haematopoietic precursor cell destruction in field cases of East Coast Fever in Tanzania. Vet Parasitol. 1994 Apr;52(3–4):243–56
- Forshaw D, Alex SM, Palmer DG, Cotter J, Roberts WD, Jenkins C, et al. Theileria orientalis Ikeda genotype infection associated with anaemia, abortion and death in beef cattle in Western Australia. Aust Vet J. 2020 Jul;98(7):290–7.
- Shiono H, Yagi Y, Chikayama Y. Oxidative damage and phosphatidylserine expression of red blood cells in cattle experimentally infected with Theileria sergenti. Parasitol Res 2003;89:228-234.
- Nassiri SM, Darvishi S, Khazraiinia P. Bovine immune-mediated hemolytic anemia: 13 cases (November 2008-August 2009). Vet Clin Path 2011;40(4): 459-466.
- Lawrence KE, Forsyth SF, Vaatstra BL, McFadden A, Pulford DJ, Govindaraju K, et al. Cluster analysis of the clinical histories of cattle affected with bovine anaemia associated with Theileria orientalis Ikeda type infection. N Z Vet J. 2017 Nov;65(6):305–12.
- Vernau W, Jacobs RM, Valli VEO, Heeney JL. The immunophenotypic characterization of bovine lymphomas. Vet Pathol. 1997;34:222–5.
- Tajeri S, Haidar M, Sakura T, Langsley G. Interaction between transforming Theileria parasites and their host bovine leukocytes. Mol Microbiol. 2021 May;115(5):860–9.