Interpretation
Marked bone marrow myelophthisis and hematopoietic hypoplasia due to a plasma cell tumor (suspect multiple myeloma).
Explanation
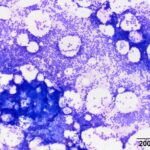
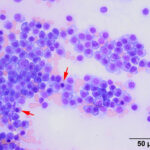
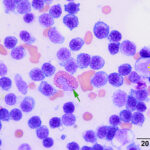
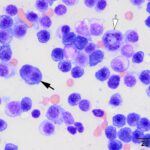
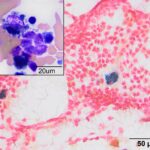
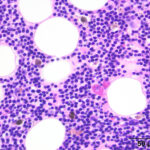
Bone marrow aspirate: The marrow aspirates were highly cellular containing multiple, densely cellular marrow particles (Figure 1) admixed with moderate amounts of free lipid (round to oval clear spaces in Figure 1) on a background of moderate amounts of blood. Approximately 93% of the marrow cells were round to slightly oval cells with moderate nuclear to cytoplasmic ratios, cobalt blue cytoplasm and often an amphophilic to lightly pink perinuclear clearance. Their nuclei were round, paracentral to eccentrically placed with clumped chromatin and indistinct nucleoli (Figures 2-3). There were low numbers of binucleated cells and rare mitotic figures (Figure 4). The round cell population resembled plasma cells (Question 2) and displayed mild to rarely moderate anisocytosis and anisokaryosis. There were low numbers of megakaryocytes peppered throughout comprised mostly of immature forms. Approximately 4% of the marrow cells were myeloid precursors with only a few band and segmented neutrophils noted, suggesting that myeloid maturation was orderly and complete. Rare eosinophil precursors (Figure 3) were also seen. The erythroid lineage was sparse (approximately 3% of total cells), consisting of rare erythroblasts, and a few rubricytes, metarubricytes (Figure 2) and polychromatophils. Erythroid maturation appeared orderly and complete. The myeloid to erythroid ratio was 1.3:1; however, this was in a marrow were only 7% consisted of residual hematopoietic tissue. A few hemosiderin-laden macrophages (Figure 5) and small dark-brown iron deposits were scattered throughout. Protein crescents were noted and, in certain areas of the smears, the background appeared basophilic (proteinaceous).
|
|
Complete Blood Count (CBC): In conjunction with the bone marrow findings, the main mechanism for the anemia (Question 1) and perhaps the thrombocytopenia is marrow myelophthisis (displacement/replacement of normal marrow hematopoietic tissue) due to a neoplastic process in this horse. Alternatively, cytokine-mediated paracrine effects could be contributing (e.g. anemia of inflammatory disease, although the tumor cells could be stimulating marrow stromal cells to secrete hematopoietic-suppressive cytokines). Although the MCV was high, which would suggest regeneration, the paucity of erythroid cells in marrow argues against this and other mechanisms could be operative, such as intramarrow folate deficiency (from usage by tumor cells) or abnormal erythroid production (albeit no overt dysplasia was seen in progenitors). The suspected presence of band neutrophils in peripheral blood suggests concurrent inflammation, likely from immunosuppression related to the tumor (the horse was reportedly neutropenic 2-weeks prior to the marrow collection). The normal neutrophil count on the repeat hemogram suggests there are focal pockets of myelopoiesis to sustain the count.
Additional tests
Due to the neurologic signs and neck stiffness, tests for equine protozoal myeloencephalitis (EPM) were requested by the veterinarian. The immunofluorescent antibody tests for Sarcocystis neurona and Neospora hughesi (both of which are implicated in EPM in horses) were negative.
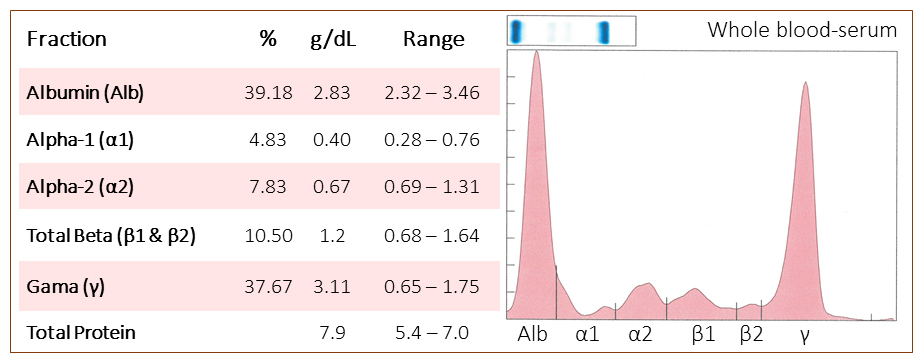
The veterinarian submitted a non-anticoagulated blood sample from the horse and serum protein electrophoresis was performed (Table 2).
The serum protein electrophoresis revealed that the hyperglobulinemia was due to a tall, sharp peak with a narrow base in the gamma region, consistent with a monoclonal gammopathy. Unfortunately, additional testing with antibodies against IgG1, IgG3/5, IgG6, IgG4/7, IgE, IgA and IgM showed normal concentrations of these proteins (multiplex testing in the Serology Laboratory in the AHDC) and a total serum IgG concentration was normal with an immunoturbidometric assay in the Clinical Pathology Laboratory. Since specific antibodies against equine IgG2 or IgGD are not available, the monoclonal protein could be one of these proteins (IgGD would be incredibly rare).
The bone marrow core biopsy revealed that the medullary spaces were filled by dense sheets of round cells admixed with scattered adipocytes and hemosiderin-laden macrophages. The round cells were 8-10 μm in diameter and had round condensed nuclei with a scant amount of cytoplasm. No mitotic activity was appreciated in this population and only a few hematopoietic precursors were identified (Figure 7). A panel of immunohistochemical stains was applied to the bone marrow core biopsy sections, including lymphocyte markers such as CD3 for T-lymphocytes and CD20, CD79α and Pax-5 for B-lymphocytes. A marker for macrophages/histiocytes, Iba1, was also applied to the core biopsy. The neoplastic population did not label with any of the applied leukocyte markers, and positive and negative controls were adequate. The histologic diagnosis was chronic lymphoproliferative disease.
Discussion
Plasma cell tumors, previously referred to reticulum cell sarcomas, are a clonal (neoplastic) proliferation of plasma cells. Plasma cells, in health, are the final and most specialized differentiation of B lymphocytes. B cells are activated and triggered to proliferate and specialize through direct recognition of foreign antigens (e.g. polysaccharides, lipid molecules) or cell-mediated interactions (i.e. cell-to-cell crosstalk and cytokine [i.e. interferon-γ, interleukin-4] release).1 Once B cells are activated, they secrete specialized proteins (i.e. immunoglobulins [Igs]) geared towards elimination and removal of the foreign elements. There are various classes of Igs, including IgA, IgE, IgG, IgD and IgM and the IgG class has 7 different isotypes. Only B lymphocytes can produce immunoglobulins. Understanding this principle is important when evaluating B cell-derived lymphoproliferative disorders, such as chronic lymphocytic leukemia or plasma cell-related diseases.
Plasma cell-related diseases is an umbrella term that encompasses plasma cell tumors (also known as plasmacytoma, extramedullary plasmacytoma), plasmablastic lymphoma, lymphoplasmacytic lymphoma and multiple myeloma among other less characterized diseases in veterinary medicine. From these, plasma cell tumors and multiple myeloma (or myeloma-related diseases), are the most common reported neoplastic processes in domestic species.2,3 Within domestic species, the different plasma cell tumors are more common in the dog.2 It has been less frequently reported in the cat and is considered unusual in horses and cattle.2 In horses, a few case reports of extramedullary plasmacytomas have been published and the tumor has been described to involve many sites, including the skin (cutaneous, subcutaneous tissues), eye, peripheral nerves, nasal mucosa, joint capsules, muscles, lymph nodes, pericardium, mediastinum, adrenal glands and bones.4–7 Within these few reports, the presence of light-chain λ-amyloid has been documented.4,6 Some authors have suggested that horses diagnosed with cutaneous amyloidosis instead have extramedullary plasmacytomas that are being overshadowed by the robust amyloid deposition.7,8 In the cases in where there was bone (i.e. third thoracic vertebrae) and nerve (i.e. trigeminal nerve) involvement, the horses were described as having neurologic manifestations, such as dysphagia and overall hind limb incoordination ranging from paraparesis to paraplegia.5,9 There has been one report of a solitary osseous plasmacytoma, however examination of other tissues for tumor involvement was not possible and it is possible that the horse had multiple myeloma (systemic or disseminated plasma cell tumors).9
Multiple myeloma, similar to other plasma cell-related neoplastic process, is a clonal proliferation of plasma cells that homes to the bone marrow. Outdated veterinary criteria require a combination of at least two of four following findings to make a diagnosis of multiple myeloma, including bone marrow plasmacytosis, monoclonal gammopathy, lytic bone lesions, and Bence-Jones proteinuria. Multiple myeloma should be considered a clinical syndrome that is an extreme variant of multiple or disseminated plasma cell tumors) that typically resides in the bone marrow. However, bone marrow infiltrates are not always reliably detected in some species and infiltration of other tissues/organs (e.g. liver, spleen) may be used to diagnose this syndrome, such as in cats. In veterinary medicine, a triggering cause for the tumor is unknown. Similarly in human medicine, an eliciting cause remains obscure and some authors suggests a familial component, while others attribute neoplastic transformation to general causes, such as irradiation or chromosomal translocation involving oncogenes, or chronic immune stimulation driving a plasma cell hyperplasia with an increased potential for malignant transformation.3,10
In the horse, multiple myeloma is a rare reported entity with only a few cases documented.10–18 The median age of affected horses is 11 years (range: 3 months – 22 years) and Quarter Horses appear to be over-represented in these reports (but are the most common breed of horse in the USA).11 Other reported equine breeds include Arabian, Thoroughbred, Morgan, American paint, Dutch Warmblood and half-bred horses. The most common reason for initial examination is weight loss, but other reported clinical signs include fever, ptyalism, limb edema and bleeding tendencies, including epistaxis, serosanguineous exudates in body cavities, and hematuria.7,10,13,19,20 Mechanisms for bleeding diathesis include thrombocytopenia due to myelophthisis, thrombocytopathia due to adherence of paraproteins to platelets and/or endothelium leading to impaired primary hemostasis and paraproteins interfering with coagulation factor function.10,19,20 Reported clinical pathologic findings include non-regenerative anemia, progressive pancytopenia, hyperproteinemia due to a monoclonal gammopathy, Bence-Jones proteinuria, and icterus, with infrequent findings of azotemia and hypercalcemia.7 Some have suggested that progressive renal disease due to chronic proteinuria from an immunoglobulin secreting plasma cell tumor is not a feature in horses due to the alkaline nature of their urine which may help prevent the formation of tubular protein casts.18 However, renal disease is still a possibility in horses with multiple myeloma.17 Hypercalcemia due to paraneoplastic production of parathyroid hormone-related protein (PTHrP) has been reported in a 13-year-old Morgan gelding with an IgA-secreting myeloma.12 The main mechanism for hypercalcemia in human patients is thought to involve localized paracrine effects that triggers osteoclastic osteolysis at sites of myeloma proliferation.21,22 Horses with multiple myeloma are at risk of infections due to multifactorial reasons, including deficiencies of non-involved immunoglobulins, impaired neutrophil function, and a defective/impaired complement system.10,19,20
In this case reported here, the morphologic features of the cells in the bone marrow aspirate on cytologic examination (which resembled plasma cells), and the presence of a monoclonal gammopathy in serum, which is >3 g/dL, supports a diagnosis of multiple myeloma. However, other expected findings including bone lysis or infiltration of extramedullary sites could not be confirmed as the horse was lost to follow-up. In horses with multiple myeloma, the most common reported paraprotein is IgG (which we suspect in this case as well), with only a few reports describing an IgA paraprotein.12,18 Rare cases of non-secretory multiple myeloma in horses have also been described.16 This horse also lacked major serum biochemical abnormalities, consistent with the majority of the reported cases.
The reason for the discrepant findings in the bone marrow aspirate (plasmacytoid features) and core biopsy (small lymphocyte features) of this case is not known, but we speculate that the dense cellularity or thickness of the core biopsy prevented the plasmacytoid features of the cells from being ascertained (the cells did have less cytoplasm than is normal for plasma cells in the bone marrow aspirate). However, it is also valid to consider the possibility that the horse did have a mature B cell neoplasm. Both mature (or small cell) B cell lymphoma and B cell chronic lymphocytic leukemia (CLL) can produce a monoclonal gammopathy (usually of IgG) in horses19,23,24 and these two spectrums of mature B cell neoplasms frequently overlap making them difficult to distinguish.23 Chronic lymphocytic leukemia is more commonly recognized in horses than multiple myeloma, but both are still rare entities in this species.19,23-25 This particular case, however, lacked the peripheral lymphocytosis that is a requirement for a diagnosis of CLL,19,23,24,25 although we cannot rule out a mature B cell lymphoma that had infiltrated the marrow (which would be unusual). The lack of immunoreactivity for common lymphocyte markers (e.g. CD3, CD20, CD79α, BLA-36) has been previously reported in horses, including mature B cell neoplasm,7,12,23,24 and, to our knowledge, MUM-1 has not been validated in the horse as a plasma cell marker. Even though there were morphologic incongruences between the bone marrow aspirate cytologic and core biopsy histologic findings in this case, morphologic discrepancies between these branches of veterinary pathology are not uncommon, because cytology is better for detailed morphologic features of cells and histopathology is better for architecture evaluation of tissues.26,27 Ultimately, taking in consideration all the aspects of this case, we can conclude that the underlying neoplastic process in this horse is a B or plasma cell tumor, either the syndrome of multiple myeloma (which fits best with the overall findings in this horse) or a mature B cell lymphoma (which does not usually extensively infiltrate marrow).
Authors: José Daniel Cruz Otero and Jacqueline Marr
References
- Kumar V. Diseases of the Immune System. Robbins Cotran Pathol Basis Dis. 2010:183-257.
- Hendrick MJ. Mesenchymal Tumors of the Skin and Soft Tissues. In: Meuten DJ, ed. Tumors in Domestic Animals. Fifth edit. John Wiley & Sons, Inc.; 2017:142-175.
- Valli VE, Bienzle D, Meuten DJ. Tumors of the Hemolymphatic System. In: Meuten DJ, ed. Tumors in Domestic Animals. Fifth Edit. John Wiley & Sons, Inc.; 2017:203-321.
- Geisell O, Grabner A, Stiglmair-Herb MT, Linke RP, Hermanns W. Kutane A-yloidose vom bei einem Pferd. Pferdeheilkunde. 1989;5(November 1988):299-304.
- Mcconkey S, López A, Pringle J. Extramedullary plasmacytoma in a horse with ptyalism and dysphagia. J Vet Diagn Invest. 2000;12:282-284.
- Higuchi T, Takase N, Matsui T. Extramedullary Plasmacytoma in a Horse. J Jpn Vet Med Assoc. 1995;48:87-90.
- Knottenbelt DC, Patterson-Kane JC, Snalune KL. Haematopoietic (round cell) neoplasms. In: Clinical Equine Oncology. First Edit. Elsevier Ltd; 2015:342-362. doi:10.1016/B978-0-7020-4266-9.00021-0
- Scott DW, Miller WH. Neoplasms, Cysts, Hamartomas, and Keratoses. In: Equine Dermatology. Second Edi. Elsevier Inc.; 2011:468-516. doi:10.1016/B978-1-4377-0920-9.00016-0
- Drew RA, Greatorex JC. Vertebral Plasma Cell Myeloma Causing Posterior Paralysis in a Horse. Equine Vet J. 1974;6(3):131-134.
- Henry M, Prasse K, White S. Hemorrhagic diathesis caused by multiple myeloma in a three-month-old foal. J Am Vet Med Assoc. 1989;194(3):392-394.
- MacAllister C, Qualls Jr C, Tyler R, Root CR. Multiple myeloma in a horse..pdf. J Am Vet Med Assoc. 1987;191(3):337-339.
- Edwards DF, Parker JW, Wilkinson JE, Helman RG. Plasma Cell Myeloma in the Horse A Case Report and Literature Review. J Vet Intern Med. 1993;7(3):169-176.
- Henry Barton M, Sharma P, LeRoy BE, Howerth EW. Hypercalcemia and high serum parathyroid hormone-related protein concentration in a horse with multiple myeloma. J Am Vet Med Assoc. 2004;225(3):409-413, 376.
- Kim DY, Taylor HW, Eades SC, Cho DY. Systemic AL Amyloidosis Associated with Multiple Myeloma in a Horse. Vet Pathol. 2005;42(1):81-84.
- Cornelius CE, Goodbary RF, Kennedy PC. Plasma Cell Myelomatosis in a Horse. Cornell Vet. 1959;49:478-493.
- Morton AJ, Davis JL, Redding WR, Jones SL. Nonsecretory multiple myeloma in a horse Case details. Equine Vet Educ. 2007;19(11):564-568. doi:10.2746/095777307X217852
- Eberhard C, Malbon A, Riond B, Schoster A. k Light-chain monoclonal gammopathy and cast nephropathy in a horse with multiple myeloma. JAm Vet Med Assoc. 2018;253(9):1177-1183.
- Pusterla N, Stacy BA, Vernau W, Cock HEVDE, Magdesian KG. Immunoglobulin A monoclonal gammopathy in two horses with multiple myeloma. Vet Rec. 2004;155:19-23.
- Jacobs RM, Kociba GJ, Ruoff WW. Monoclonal Gammopathy in a Horse with Defective Hemostasis. Vet Pathol. 1983;20:643-647.
- Schalm OW, Knight HD, Osburn BI. Idiopathic gammopathy and plasmacytosis in a horse. Calif Vet. 1974.
- Mundy GR, Raisz LG, Cooper RA, Schechter GP, Salmon SE. Evidence for the Secretion of an Osteoclast Stimulating Factor in Myeloma. N Engl J Med. 1974;291(20):1041-1046.
- Saeki Y, Mima T, Ishii T, et al. Enhanced production of osteopontin in multiple myeloma : clinical and pathogenic implications. Br J Haematol. 2003;123:263-270.
- Badial PR, Tallmadge RL, Stokol T, Felippe MJ. Applied protein and molecular techniques for characterization of B cell neoplasms in horses. Clin Vaccine Immunol. 2015;22(11):1133-1145.
- Dascanio JJ, Zhang CH, Antczak DF, Blue JT, Simmons TR. Differentiation of Chronic Lymphocytic Leukemia in the Horse A Report of Two Cases. J Vet Intern Med. 1992;6(4):225-229.
- Long AE, Javsicas LH, Stokol T, Felippe MJB, Frimberger AE. Rapid Clinical Progression of B-cell Chronic Lymphocytic Leukemia in a Horse. J Am Vet Med Assoc. 2019;255(6):716-721.
- Gilotra M, Gupta M, Singh S, Sen R. Comparison of bone marrow aspiration cytology with bone marrow trephine biopsy histopathology : An observational study. J Lab Physicians. 2017;9(3):182-189. doi:10.4103/JLP.JLP
- Gal A, Burchell RK, Worth AJ, Lopez-Villallobos N, Marshall JC, MacNeill AL. The Site of Bone Marrow Acquisition Affects the Myeloid to Erythroid Ratio in Apparently Healthy Dogs. Vet Pathol. 2018;55(6):853-860. doi:10.1177/0300985818780469