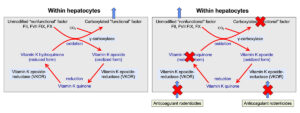
When coagulation factors are synthesized in the liver, they are non-functional. They need to be modified (by gamma-carboxylation of glutamic acid residues in the N-termini) to become functional, i.e. capable of binding calcium and phospholipid membranes. This is accomplished by an enzyme in hepatocytes called gamma-carboxylase (because it carboxylates glutamic acid residues in the factors, by adding carbon dioxide). Vitamin K (in the reduced form) acts as a cofactor for the carboxylation reaction and, in the process, becomes oxidized, which is called the vitamin K epoxide.
Vitamin K epoxide (oxidized form) must be recycled back to its reduced form to work in the carboxylation reaction. This is accomplished by a vitamin K epoxide reductase or vitamin K oxidoreductase (VKOR) (left panel). The latter enzyme is inhibited by anticoagulant rodenticides as shown above in the right panel and blocks both reduction steps. Other enzymes (which are not inhibited by the anticoagulant rodenticides) may also reduce the quinone form of vitamin K to the fully reduced hydroquinone form, but VKOR is still required for the first reduction step.
Lack of vitamin K or absence of cycling of vitamin K (from oxidized to reduced forms) due to inhibition by rodenticides prevents modification and functionalization of the vitamin K-dependent factors. The inactive factors are secreted into the circulation (instead of the carboxylated or “functional” versions) and are called Proteins Induced by Vitamin K Antagonism or Absence (or PIVKAs). The animal therefore lacks functional coagulation factors (II, VII, IX, X), resulting in prolonged PT and aPTT and clinical hemorrhage. The PT usually prolongs before the aPTT, because of the shorter half life of FVII, however, dogs usually only bleed when the aPTT is prolonged due to deficient FX or prothrombin.