Interpretation
Injection/vaccine-induced panniculitis characterized by mixed, predominantly macrophagic inflammation (answer to question 1).
Explanation
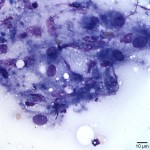
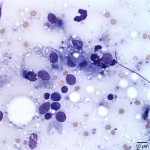
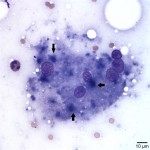
The examined slides were comprised of many inflammatory cells within a background of fat and proteinaceous material. Most of the inflammatory cells consisted of macrophages (Figure 1 and Figure 2), with fewer numbers of lymphocytes and rare non-degenerate neutrophils . Moderate numbers of giant multinucleated macrophages were seen (Figure 3). Many macrophages, including the multinucleated forms, were packed with indistinct globules of pale to medium purple protein material in their cytoplasm. This material was presumed to be vaccine-derived adjuvant (answer to question 2) and was also observed extracellularly as amorphous globules. Lymphocytes were mostly small, mature forms. Low numbers of plasma cells and focal areas of necrosis were also observed (not shown).
Taken together with clinical history and signalment, these cytologic findings were most compatible with injection/vaccine-induced panniculitis (answer to question 3).
Discussion
Panniculitis consists of inflammation of subcutaneous fat (panniculus). It is uncommonly diagnosed in the cat and rarely in the dog and it can be infectious or noninfectious.1,2 Nonifectious etiologies include immune-mediated conditions and pancreatic disease, vasculopathy, trauma, foreign body, drug-eruption, vitamin E deficiency, injection-induced, and idiopathic.2 Idiopathic panniculitis is probably the most common noninfectous form. However, injection-induced panniculitis is probably underdiagnosed, since lesions may not be obvious clinically and might be deemed inconsequential. 2,3
Injection-induced panniculitis is caused predominantly by vaccines, and less commonly by other injected medications.1 The reaction may occur within days to several weeks after injection and it appears in the cat and dog as a solitary nodule which varies in diameter (from 5 mm to 3 cm). It can be freely movable or fixed to the overlying dermis and varies from fluctuant to firm.1,4 The overlying skin is commonly normal, but focal alopecia with minimal gross inflammation may occasionally occur (particularly after Rabies vaccination).1,2 Lesions are usually non-painful, but pain may be elicited on palpation.1 The condition is suspected if the lesion presents on a site of previous injection, but skin biopsy is necessary to confirm the diagnosis.1
The vaccine component most commonly associated with post-vaccine inflammation is the adjuvant.5 Most inactivated vaccines contain an adjuvant which enhances the inflammation at the site of injection, consequently triggering the desired immune response.6 Potential adjuvant materials include aluminum in the form of aluminum hydroxide or aluminum phosphate, oil emulsions, saponins, or polysaccharide derivatives.5,7
No breed, age or sex predilection has been reported for injection-induced panniculitis.1 Although the exact etiology remains unclear, the histopathologic pattern of these types of lesions suggests a combined foreign body and hypersensitivity reaction to vaccine or other injected medications.1,4,2 The majority of panniculitis are granulomatous or pyogranulomatous.3The epidermis and dermis are usually normal, but inflammation may extend to the deep dermis.1 The panniculus contains discrete nodules, often with a central zone of necrosis. Particularly in reactions due to vaccines, the central zone of necrosis often contains granular to elongated aggregates of basophilic material, presumably derived from vaccine adjuvant or other vaccine components. Prominent lymphoid follicle formation may be seen on the perimeter of the lesion admixed with marked eosinophilic inflammation.1
Cytologically, injection-induced panniculitis is characterized by moderately cellular aspirates comprised of mixed inflammatory cells in a thin proteinaceous background containing free lipid.4,8 There are large amounts of nuclear and cellular debris with variable numbers of macrophages, giant multinucleated cells and non-degenerate neutrophils. Small lymphocytes and plasma cells are present, may be numerous and correspond to lymphoid follicles that can be seen on the periphery of these lesions during histopathologic examination. Variable numbers of eosinophils and few spindle cells may be present.1,4,8 Often, abundant pink to purple, to sometimes blue- to gray-staining material (as seen in this case), ranging from amorphous to globular is present both extracellularly and within macrophages.4,8 This material which is similarly seen on histopathology (as described above) is likewise presumed to be vaccine-derived. The origin of this blue-gray material as being vaccine-derived was further confirmed by a recent study. This study evaluated the staining properties of a similarly described material observed in vaccine-induced lesions and showed that the material may be metal-containing adjuvant.7 The blue-gray material observed in some of the examined vaccine-induced lesions was positive for Morin stain, a fluorescent stain that reacts with some metal ions, and is often used to highlight aluminum.7
In this cat, the results were most compatible with vaccine-induced panniculitis. There was no evidence of neoplasia in the aspirate. However, neoplastic transformation, particularly in cats, can occur in this type of lesions and can be intersperse with zones of inflammation. Neoplastic cells, therefore can be missed on aspiration. Neoplastic transformation is suspected if the mass persists for more than three months after vaccination, if the mass becomes larger than 2 cm in diameter, or is increasing in size 1 month after vaccination.5,4 Hence the importance of post-vaccination monitoring. The etiology of malignant transformation is likely multifactorial, but overzealous inflammation seems to be the most important predisposing factor of malignant transformation in susceptible cats.5,6,9,10
References
- Gross TL, Ihrke PJ, Walder EJ, Affolter VK: Skin diseases of the Dog and Cat, 2nd ed., pp. 538-542. Blackwell Publishing Professional, Ames, IA, U.S.A. 2005
- Ariane N. Panniculitis in dogs and cats. Companion Animal. 2011;16: 27-28
- Ginn PE, Mansell JEKL, Rakich PM: Skin and appendages. In: Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 1, pp. 573-574. Elsevier Limited, St. Louis, MO, 2007
- Fisher DJ. Cutaneous and subcutaneous leiosn. In: Cowell R and Tyler R, ed. Diagnostic Cytology and Hematology of the Dog and Cat. 4th ed. St. Louis, MO: Elsevier Mosby, 2013:91-92.
- Dennis WM. Current understanding of vaccination site-associated sarcoma in the cat. Journal of Feline Medicine and Surgery. 1999;1: 15-21
- Hartmann Katrin. et al. Feline injection-site sarcoma. ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery. 2015;17: 606-613
- Jennifer L. et al. Identification of blue staining vaccine-derived material in inflammatory lesions using cultured canine macrophages. Veterinary Clinical Pathology. 2015;1:152-156
- Raskin ER. Skin and subcutaneous tissues. In: Raskin ER, Meyer DJ, eds. Canine and Feline Cytology, A Color Atlas and Interpretation Guide. 2nd ed. St Louis, MO: Saunders Elsevier; 210:28-29
- Margaret CM and Rodney LP. Feline Associated Sarcomas. Journal of Veterinary Internal Medicine. 2001;16:176-182
- Jolle K. Feline injection site-associated sarcoma: Is it a reason to critically evaluate our vaccination policies? Veterinary Microbiology. 2006;117:59-65
Authors: D Hernandez (clinical pathology resident)