Interpretation
Inflammation with acute kidney injury, liver injury, and cholestasis
Explanation
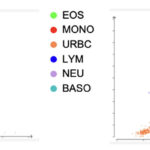
Based on the automated CBC results, there is an inflammatory leukogram, consisting of a moderate leukocytosis due to a moderate mature neutrophilia and moderate monocytosis. There is also a marginal basophilia. When interpreting WBC dot plots there should be a clear distinction between clusters – when this does not happen and the clusters overlap, it indicates the instrument had difficulty identifying the cells correctly. On this patient’s dot plot there is a region of overlapping colors encompassing monocytes, lymphocytes, basophils, and neutrophils (Figure 1), suggesting there may be misclassification of these cells. A common scenario where these three cells are misclassified is when there is a left shift in neutrophils. A left shift indicates there are immature neutrophils in the blood. This can be problematic for the instrument because immature neutrophils can be erroneously identified as lymphocytes and/or monocytes, especially if there are metamyelocytes present in addition to band neutrophils. Furthermore, a left shift is often accompanied by toxic changes in neutrophils. Toxic change increases fluorescence of neutrophils, resulting in these cells being shifted higher along the y-axis where lymphocytes and monocytes are typically located and classified. This misclassification of immature or toxic neutrophils as monocytes and/or lymphocytes can lead to an incorrect differential count from the instrument, with falsely lowered neutrophil counts and falsely increased monocyte and/or lymphocyte counts. Thus, the overlapping clusters observed on this dot plot warrants a blood smear analysis to determine a more accurate leukocyte differential count, including the presence and severity of a left shift and toxic change and verification of the monocytosis and basophilia (Question 1). A blood smear examination and manual differential cell count was not done in this case.
Given the predominant findings of severe azotemia and increased liver enzymes, compatible with liver injury (ALT) and cholestasis (ALP) in the biochemical panel, along with the history of exposure to an outdoor backyard and lack of relevant vaccination, a leptospiral infection was considered as the top differential diagnosis. The high ALP activity was attributed to cholestasis and not only the young age of the dog (even though a total bilirubin concentration was not measured), because the ALP is increased to a higher degree than that expected from the bone isoform due to age and the puppy was visibly icteric. Pancreatitis would be an alternative diagnosis, but uncommon (as is leptospirosis) in such a young puppy (Question 2). Acute kidney injury (AKI) is one of the most common manifestations of leptospirosis, where the bacterial organisms infiltrate the kidney tissue and cause acute tubulointerstitial nephritis. As the name implies, tubulointerstitial nephritis results from inflammation in the renal tubules and the tissue that surrounds them. The glomeruli are responsible for filtering waste while the renal tubules reabsorb vital filtered substances and are required for dilution and concentration of the urine. In acute tubulointerstitial nephritis, loss of glomerular function results in azotemia and the normal tubular reabsorption process is disturbed. As a result, a urinalysis from a patient with leptospirosis can show indicators of tubular damage including glucosuria (with normoglycemia), excessive proteinuria for the urine specific gravity, and isosthenuria (inability to concentrate or dilute urine), due to defective resorption of glucose and proteins and tubular concentrating ability, respectively. Granular or cellular casts may be seen due to the tubular injury and there may also be pyuria and hematuria from renal inflammation.1 Liver dysfunction is another common manifestation of leptospirosis and results from bacterial invasion of the liver, leading to hepatocellular damage and bile reflux into the circulation.1 Therefore, bilirubinuria (from filtering of the conjugated bilirubin) is an expected finding in this patient’s urinalysis, given the suspected cholestasis. Although Leptospira are shed in urine, their width is too small for the resolution of light microscopy so they are not visible in the urine sediment.1
Follow up and outcome
A point-of-care Canine Leptospira Antibody ELISA snap test was performed and was positive for leptospirosis. Given the lack of a leptospirosis vaccine history, a false positive reaction was considered unlikely. Additional testing to help confirm the diagnosis and hospitalization were recommended, but due to financial limitations, no further testing was pursued and the owner declined hospitalization. Treatment with doxycycline and supportive care, such as anti-emetics, was attempted on an outpatient basis for a presumptive leptospirosis infection, however the patient passed away at home.
Discussion
Leptospirosis is a zoonotic bacterial infection caused by gram-negative, aerobic, flagellated spirochetes belonging to the genera Leptospira.1 There are 68 known species of Leptospira that can be divided into two groups based on their DNA sequencing: “pathogenic” and “saprophytic” groups.2,3 Within the pathogenic group, there are over 300 serovars that can be serotyped into antigenically-related serogroups based on their outer lipopolysaccharide antigens.3 Leptospira are shed in the urine of infected host animals, which serve as reservoirs, and contaminate water and soil, remaining there for weeks to months.1,4 The bacteria thrive in warm, wet environments so they are more prevalent in warmer climates with periods of heavy rainfall.1 While any wildlife or domestic animal can be a reservoir host, rodents are by far the most common species.5 Although prevalence varies by region and season, all dogs are considered at risk for becoming infected by leptospirosis.1 By contrast, cats are rarely reported to suffer from clinical disease, even if there is serologic evidence of an active leptospirosis infection, and thus they can be considered another reservoir host.6,7
An animal or person becomes infected with Leptospira by direct (e.g., bite wounds, eating infected meat etc.) or indirect contact with contaminated sources (e.g., water, soil, bedding, etc.), after which a pathogenic strain enters the bloodstream and replicates rapidly (3-10 days) and then invade other organs, such as the kidney and liver.1,8 The organism is shed into urine between 7-14 days after infection.1 Leptospirosis can range from a mild infection with no clinical signs to a severe infection with multiple-organ failure and death.1 Although the disease is multisystemic, the two most prominent findings are renal and hepatic dysfunction, which usually manifest together.1,9 Clinical signs usually manifest within 7 days of infection. Renal impairment is caused by the spirochetes invading the kidney tissue leading to acute tubulointerstitial nephritis.1,10 Liver dysfunction results from the spirochetes causing hepatocellular injury and death and likely cholestasis, although disruption of transcellular boundaries can also lead to biliary reflux into the circulation.1 Other possible clinical signs include pulmonary hemorrhage, excessive bleeding due to coagulopathies, vasculitis, uveitis, pancreatitis, and muscle pain.1,11,12
Serum biochemistry results in infected patients depend on the organs affected and the level of dysfunction at that point in time. Acute kidney injury manifests azotemia (increased concentrations of creatinine, urea nitrogen), hyperphosphatemia from decreased glomerular filtration rate and isosthenuria.1,13 Potassium concentrations can be increased or decreased as a result of impaired tubular sodium reabsorption affecting potassium secretion, with hypokalemia reported in dogs with non-oliguric AKI and hyperkalemia in those with oliguric or anuric AKI.1,13 Urinalysis typically shows indicators of tubular damage, as described above for Question 3.1,14 Hepatic injury manifests with increased ALT, AST and ALP activities, with the ALP activity often being increased to a greater degree than the ALT and AST activities.1 The reason for this is unclear, but may be related to a cytokine-induced (functional) cholestasis or potentially direct induction of ALP synthesis. Electrolyte abnormalities frequently accompany leptospirosis infections and include hypochloremia and hyponatremia, as seen in this case.1,14 A corrected chloride was normal in the dog of this reportr (109 mEq/L), suggesting the changes in chloride were due to electrolyte losses without an additional acid-base abnormality. Hemogram results typically demonstrate a leukocytosis characterized by a neutrophilia, often with a left shift, and monocytosis, lymphopenia, thrombocytopenia (thought to be cytotoxic effects of the organism), and a mild to moderate non-regenerative anemia. The anemia can be severe if there is concurrent pulmonary or gastrointestinal hemorrhage or coagulopathies.14,15 There can be laboratory evidence of disseminated intravascular coagulation, including prolonged screening coagulation times (prothrombin and activated partial thromboplastin times), low antithrombin concentration, and increased D-dimer concentration. Fibrinogen concentration cabn be normal, increased, or decreased depending on the balance between inflammation (increasing production) and consumption (decreasing values).14,15
The diagnosis of leptospirosis is not straight-forward and is made on the basis of clinical suspicion (any animal with AKI with or without pulmonary or liver disease should be tested for the organism) and diagnostic testing.1 There are many tests available for leptospirosis but serologic- and genetic-based polymerase chain reaction (PCR) assays predominate, with cultures being less common. All have limitations, so a combination of tests is recommended to confirm the diagnosis.
- Microscopic agglutination test (MAT): This serologic test is based on the detection of Leptospira antibodies in blood. It is a quantitative test that has high specificity and low sensitivity16 and is performed by incubating dilutions of the patient’s serum with various Leptospira serovars. These serovars should be representative of those common to the region – in veterinary medicine this is typically six to seven serovars, but in human medicine, the test can include >30 different serovars.1,16 After incubation, the samples are inspected by dark-field microscopy for agglutination, and the highest serum dilution resulting in 50% agglutination is the “acute-phase” titer reported to the clinician. It is not recommended to make a diagnosis of leptospirosis based on a single positive MAT result, so another MAT should be performed 7–14 days later, which is considered the “convalescent-phase” titer.1 Seroconversion (based on a ≥ 4-fold rise) in the second titer indicates a current leptospirosis infection.16 This is a limitation, because the time it takes to perform each MAT and having to submit a second titer for confirmation delays the diagnosis and potential treatment, if relying solely on MAT results. Other limitations to the test include false negatives if tested too early in infection (insufficient antibodies produced), antibiotics are given before the immune system has responded appropriately, or wrong serovars are included.1,16 Alternatively, the test can produce false positives if there had been recent subclinical exposure or after a leptospirosis vaccine.16 In addition, the infecting serovar cannot be reliably identified with the MAT, because of a high degree of cross-reactivity between serovars.16
- Point-of-Care serologic tests: There are other serologic tests that are available as point-of-care, in-clinic tests for rapid detection of Leptospira antibodies licensed in the USA.17 These tests are qualitative, producing a “positive” or “negative” result, with varying sensitivity and specificity depending on the local serovars.1,17 One test works by detecting the patient’s IgM antibodies to multiple Leptospira antigens and the other by detecting antibodies to the lipoprotein LipL32 on the outer membrane of pathogenic Leptospira species.17 These tests can be false negative or positive in the same situations as the MAT and also do not identify the infecting serovar.
- Genetic testing: Genetic testing is based on detection and amplification of Leptospira DNA in blood and/or urine (also CSF and other tissues, although these tissues are used less commonly) using specific primers. This is a qualitative test with variable sensitivity and specificity, which is related to natural progression of the disease (early blood infection followed by later urine shedding) and tested sample type.18 Thus, ideally blood and urine should be submitted for PCR during the initial (first 7 days) and second (>7 days) phases, respectively, however since the precise timing of infection is unknown, it is recommended to submit both samples for PCR testing.1 These assays have a rapid turnaround and detect early infections before antibodies are produced.18 A study of 20 dogs after vaccination did not yield positive PCR results, although the vaccine DNA was detected when spiked into canine whole blood.19 DNA PCR assays can produce false negative results with prior antibiotic therapy as Leptospira are readily killed by antibiotics leaving too few bacteria to detect the infection.18 In rare cases, a PCR assay can yield a false positive result when the patient has been exposed to a type of Leptospira that is non-pathogenic.18 Like serologic tests, they do not determine the infecting serovar.18
- Culture: Bacterial cultures can be performed from samples such as blood, urine, or other tissues and is the only method that can definitively identify the infecting Leptospira serovar.1 Culturing requires inoculation of a special media using a fresh sample at point-of-care and before the administration of antibiotics.1 Bacterial cultures require substantial expertise for sample handling and interpretation and tend to have low sensitivity.1 Additionally, many Leptospira serovars require long culture periods of up to 13 weeks.20 As such, cultures are not regularly used in the diagnosis of leptospirosis for individual patients. However, epidemiologically, cultures are still valuable when attempting to identify the serovars in various geographical locations.20
Treatment of leptospirosis entails antimicrobial therapy to target the Leptospira bacteria, with doxycycline being the antibiotic of choice, as well as supportive care for underlying tissue injury.1 Depending on the clinical signs and degree of organ dysfunction, treatments can range from simple outpatient supportive care to intensive inpatient hospitalizations with extreme interventions such as dialysis for AKI. Treatment should not be delayed while awaiting for MAT or PCR results and should begin immediately after blood and urine samples have been collected. Thus, point-of-care testing can be valuable in directing and immediately initiating treatment in affected patients.
Vaccines are available for the protection against leptospirosis infection in many animals, including dogs, horses, cattle, sheep, goats, pigs, and deer. Currently there are no vaccines approved for cats. Leptospira vaccines differ by serogroup content and can be monovalent, bivalent, trivalent, or quadrivalent.1 In North America, the current quadrivalent vaccines for dogs contain the serovars Canicola, Grippotyphosa, Icterohaemorrhagiae, and Pomona; in Europe they include Canicola, Grippotyphosa, Icterohaemorrhagiae, and Australis.1 It remains unknown if an animal acquires immunity to leptospirosis or if there is cross-protection between serovars after surviving an infection with one serovar, so until the antibacterial immune response is better understood, it is recommended to vaccinate any patient as soon as possible after their recovery.1
Author: Colette Angel DVM; edited by T Stokol.
References
- Sykes JE, Francey T, Schuller S, Stoddard RA, Cowgill LD, Moore GE. 2023 Updated ACVIM consensus statement on leptospirosis in dogs. J Vet Intern Med. 2023; 1–17.
- List of prokaryotic names with standing in nomenclature (LPSN). Genus Leptospira. https://www.bacterio.net/genus/Leptospira. Accessed 2023.
- Vincent AT, Schiettekatte O, Goarant C, et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospirathrough the prism of genomics. PLoS Negl Trop Dis. 2019; 13(5).
- Yanagihara Y, Villanueva S, Nomura N, et al. Leptospirais an environmental bacterium that grows in waterlogged soil. Microbiol Spectr. 2022; 10(2).
- Boey K, Shiokawa K, Rajeev S. Leptospirainfection in rats: a literature review of global prevalence and distribution. PLoS Negl Trop Dis. 2019; 13(8).
- Mazzotta E, De Zan G, Cocchi M, et al. Feline susceptibility to leptospirosis and presence of immunosuppressive co-morbidities: first European report of interrogansserogroup Australis Sequence Type 24 in a cat and survey of Leptospira exposure in outdoor cats. Trop Med Infect Dis. 2023; 8(1):54.
- Murillo A, Goris M, Ahmed A, Cuenca R, Pastor J. Leptospirosis in cats: current literature review to guide diagnosis and management. J Fel Med Surg. 2020; 22(3):216–228.
- Surdel MC, Anderson PN, Hahn BL, Coburn J. Hematogenous dissemination of pathogenic and non-pathogenic Leptospirain a short-term murine model of infection. Front Cell Infect Microbiol. 2022; 12.
- Rahman SA, Khor KH, Khairani-Bejo S, et al. Detection and characterization of Leptospira in dogs diagnosed with kidney and/or liver disease in Selangor, Malaysia. J Vet Diagn Invest. 2021; 33(5):834–843.
- Abdulkader RC, Silva MV. The kidney in leptospirosis. Pediatr Nephrol. 2008; 23(12):2111–2120.
- Kohn B, Steinicke K, Arndt G, et al. Pulmonary abnormalities in dogs with leptospirosis. J Vet Intern Med. 2010; 24(6):1277–1282.
- Wollanke B, Gerhards H, Ackermann K. Infectious uveitis in horses and new insights in its leptospiral biofilm-related pathogenesis. Microorganisms. 2022; 10(2):387.
- Birnbaum N, Barr SC, Center SA, Schermerhorn T, Randolph JF, Simpson KW. Naturally acquired leptospirosis in 36 dogs: serological and clinicopathological features. J Small Anim Pract. 1998; 39: 231–236.
- Knopfler S, Mayer-Scholl A, Luge E, et al. Evaluation of clinical, laboratory, imaging findings and outcome in 99 dogs with leptospirosis. J Small Anim Pract. 2017; 58(10):582–588.
- Barthelemy A, Viole A, Cambournac M, et al. Hematological and hemostatic alterations associated with a single extracorporeal renal replacement therapy in dogs with acute kidney injury associated leptospirosis: a pilot study. Top Companion Anim Med. 2020; 38.
- Goris MG, Hartskeerl RA. Leptospirosis serodiagnosis by the microscopic agglutination test. Curr Protoc Microbiol. 2014; 32.
- Lizer J, Velineni S, Weber A, Krecic M, Meeus P. Evaluation of 3 serological tests for early detection of Leptospira-specific antibodies in experimentally infected dogs. J Vet Intern Med. 2018; 32(1):201–207.
- Martin EA, Heseltine JC, Creevy KE. The Evaluation of the Diagnostic Value of a PCR Assay When Compared to a Serologic Micro-Agglutination Test for Canine Leptospirosis. Front Vet Sci. 2022; 9.
- Midence JN, Leutenegger CM, Chandler AM, Goldstein RE. Effects of Recent LeptospiraVaccination on Whole Blood Real-Time PCR Testing in Healthy Client-Owned Dogs. J Vet Intern Med. 2012; 26(1):149–152.
- Adler B, De la Peña-Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010; 140(3-4):287–296.
