Peritoneal fluid analysis & cytologic findings
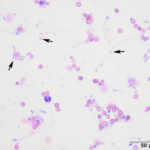
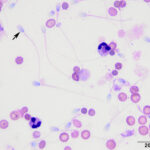
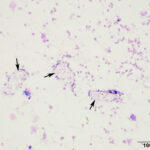
The peritoneal fluid was of low cellularity and the background was clear to lightly stippled. The sample contained low numbers of intact and lysed erythrocytes admixed with low to moderate numbers of small (approximately 3 µm x 6 µm) oval shaped sperm with long tails (up to 40 µm) (Question 1). A few neutrophils and rare macrophages were noted. Neutrophils were non-degenerate to mildly degenerate with low numbers undergoing karyorrhexis and karyolysis. Macrophages were both non- and lightly-vacuolated. Within the cytospin smears, there were a few clear, degrading polygonal- to rectangular-shaped urinary crystals (Figure 4), presumably struvite. There was a small amount of necrotic cellular debris. No infectious organisms were observed.
Case interpretation
Uroabdomen secondary to blunt trauma (Question 2)
Additional tests
Although the presence of sperm and urinary crystals in the abdomen of a dog was diagnostic in itself, the diagnosis was confirmed by measurement of creatinine concentrations in the peritoneal fluid and serum (Question 3). The peritoneal fluid creatinine was 173.4 mg/dL, leading to an effusioncreatinine /serumcreatinine ratio of 216.8 (>2:1 indicates a uroabdomen).
Discussion
Uroabdomen is defined by the presence of urine within the abdominal cavity.1-5 The diagnosis of uroabdomen (also known as uroperitoneum) in this case was readily achieved by the microscopic identification of spermatozoa within the effusion. The presence of urinary crystals (Figure 4) added additional confirmation that a region within the genitourinary tract was disrupted during or after the traumatic event. The persistent hyperglycemia was attributed to chronic stress from endogenous glucocorticoids. Transient contributions from epinephrine cannot be ruled out. Muscle injury from the traumatic event was considered the cause for the changes in AST and CK activities. The mild hyperlactatemia at presentation indicated type 1 lactic acidosis, from hypovolemia or hypoperfusion (post-traumatic shock)-induced anaerobic metabolism. Fluid shifts into the peritoneal space, resulting in hypovolemia, is primarily attributed to a creatinine-induced concentration gradient.6
In dogs, and similarly in cats, the most common site of genitourinary disruption is the urinary bladder, followed by the urethra.2–5 Other less common sources are the kidney and ureter.2–4 Uroabdomen in dogs and cats is typically attributed to trauma, either iatrogenic (i.e., excessive palpation/forced expression of the urinary bladder, secondary to catheterization and/or surgical intervention) or associated with blunt traumatic events.2–5 Urinary obstruction from urinary calculi or cancer are also reported causes for a uroabdomen.4,5,7 The history and clinical findings, along with biochemical assessment of body cavity effusions and contrast imaging (i.e., cystourethrography, excretory urography) can be used to facilitate the diagnosis.5,7 Intra-operative visualization of a rupture within the genitourinary system has been documented.4 Biochemical analysis has been proven to be a highly sensitive and specific test for the diagnosis of uroabdomen, when at least 2 of the following criteria are met; The creatinine concentration of the effusion is >4 times the upper limit of the RI for serum creatinine, an effusion/serum creatinine ratio is > 2.0, or an effusion to serum potassium ratio is >1.4.8 In the case presented here, two of these criteria were met.with the effusion creatinine (173.4 mg/dL) concentration being 129 times greater than the upper limit of the RI for serum creatinine (1.4 mg/dL) and the effusion to serum creatinine ratio (216.8) being >2.0. In addition, a ureteral tear at the level of the prostate was confirmed with a positive contrast cystourethrogram.
The cytologic characteristics of the fluid (gross features, protein and nucleated cell counts) taken alone are consistent with a low-protein transudate. However, this classification is applied to an effusion caused from alterations in hydrodynamic forces versus that due to a urinary tract rupture, where the abdomen is flooded or diluted by low protein urine. Thus, the traditional classification of a low-protein transudate does not really fit, other than as a descriptive term, i.e. it is not mechanistic. Low protein abdominal fluids are typical of acute uroabdomen.1–3 With time, the effusion shifts towards an exudate (nucleated cell count: > 5,000 cells/µL, dominated by neutrophils, total solids: > 3.0 g/dL), due to chemical irritation of serosal surfaces (peritonitis) by the urine.2,3,5 Regardless of chronicity, urine generates a hostile environment and inflammatory and tissue resident cells (i.e., neutrophils, macrophages, mesothelial cells, etc) within the fluid can often appear poorly preserved and degraded.2,3 Similar to the few neutrophils in the effusion of this case, neutrophils are often described to display features of cell death, including pyknosis, karyolysis and karyorrhexis.2,3 Septic peritonitis can occur in patients with concurrent urinary tract infections,5 but was not present in this patient. Therefore, in cases of uroabdomen, it might be worthwhile to collect samples for aerobic and anaerobic bacterial cultures, since cytologic evaluation of effusions alone may not always detect a septic process (e.g. if only low numbers of bacteria are present).
Patients with uroadomen often require initial stabilization with fluid resuscitation and placement of a urinary catheter.5 Peritoneal drainage via catheters is often required to remove the caustic irritant and perhaps for implementation of peritoneal dialysis.4,5 The need for surgical intervention to repair the tear depends on the severity and location of the injured tissue.5 For example, medical management with placement of indwelling urinary catheters alone can be used to manage urethral tears, without surgical intervention.5,9 The damaged urinary epithelium is estimated to regenerate within 7 days.9 Advanced surgical techniques, such as nephrostomy tube placement and subcutaneous ureteral bypass, have been documented in veterinary medicine, but their success appears to be patient-dependent.10
The survival rate of dogs with uroabdomen is good, with 79% of dogs in one study surviving to hospital discharge,4 consistent with a previous report.7 Serum creatinine concentrations at admission were not predictive of the outcome.
Unfortunately, due to financial constraints, the dog in this case was humanly euthanized after medical management with pain medication, antibiotic therapy, strict cage rest and placement of an indwelling urinary catheter were attempted. This case highlights the value of cytologic assessment for the diagnosis of uroadomen. The presence of spermatozoa within the effusion of this patient was an unexpected but diagnostic finding, as was the presence of crystals typically seen in urine.
References
- Stockham SL, Scott MA. Cavitary Effusions. In: Fundamentals of Veterinary Clinical Pathology. 2nd ed. Ames, Iowa: Blackwell Publishing; 2008. p. 831–68.
- Valenciano AC, Arndt TP, Rizzi TE. Effusions: Abdominal, Thoracic, and Pericardial. In: Diagnostic Cytology and Hematology of the Dog and Cat. 4th ed. St. Louis, Missousi: Elsevier Inc.; 2014. p. 244–65.
- Thompson CA, Rebar AH. Body Cavity Fluids. In: Canine and Feline Cytology [Internet]. Third Edit. St. Louis, Missousi: Elsevier Inc.; 2016. p. 191–219. Available from: http://dx.doi.org/10.1016/B978-1-4557-4083-3.00006-1
- Grimes JA, Fletcher JM, Schmiedt CW. Outcomes in dogs with uroabdomen: 43 cases (2006-2015). J Am Vet Med Assoc. 2018;252(1):92–7.
- Stafford JR, Bartges JW. A clinical review of pathophysiology, diagnosis, and treatment of uroabdomen in the dog and cat. J Vet Emerg Crit Care. 2013;23(2):216–29.
- Rieser TM. Urinary tract emergencies. Vet Clin North Am – Small Anim Pract. 2005;35(2 SPEC. ISS.):359–73.
- Anderson RB, Aronson LR, Drobatz KJ, Atilla A. Prognostic factors for successful outcome following urethral rupture in dogs and cats. J Am Anim Hosp Assoc. 2006;42(2):136–46.
- Schmiedt C, Tobias KM, Otto CM. Evaluation of Abdominal Fluid: Peripheral Blood Creatinine and Potassium Ratios for Diagnosis of Uroperitoneum in Dogs. J Vet Emerg Crit Care. 2001;11(4):275–80.
- Boothe HW. Managing traumatic urethral injuries. Clin Tech Small Anim Pract. 2000;15(1):35–9.
- Berent AC. Ureteral obstructions in dogs and cats: A review of traditional and new interventional diagnostic and therapeutic options. J Vet Emerg Crit Care. 2011;21(2):86–103.
Author: José Daniel Cruz Otero