Interpretation
Mesenchymal tumor
Explanation
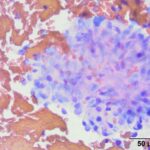
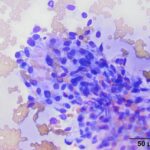
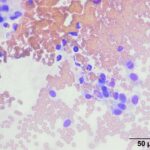
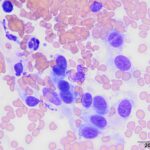
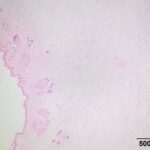
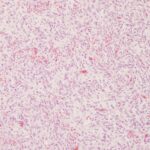
The submitted smears contained several aggregates of spindle cells (Question 1), caught up within a light pink to purple extracellular matrix (Figures 1-2a). The spindle cells had round to oval central nuclei with lacy chromatin and 1-2 small indistinct nucleoli. The cells contained a moderate amount of amphophilic cytoplasm that tapered away from both poles of the nuclei. Cytoplasmic borders were indistinct and wispy to veil-like in many cells. The cells displayed minimal to mild anisocytosis and anisokaryosis (Figure 1-4a).
The aggregates of the cells, with their cytologic features, were reminiscent of cells aspirated from soft tissue sarcomas of dogs, many of which are nerve sheath tumors. A cutaneous nerve sheath tumor was considered the top differential diagnosis in this case, although a tumor of synovial origin or other mesenchymal tumor could not be ruled out (Question 2).
Additional tests
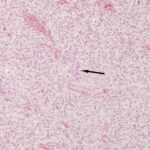
An excisional biopsy was performed and formalin-fixed tissue (3.0 x 2.1 x 1.6 cm mass with sparse hair) was submitted for histopathologic evaluation. Slides prepared from paraffin-embedded sections of the mass were routinely stained with hematoxylin and eosin. Histologic examination revealed an expansile, variably cellular, poorly demarcated mass infiltrating and expanding the dermis and subcutis. The mass was composed of thin spindle cells arranged in two distinct patterns. Approximately 60 percent of the mass consisted of cells organized in densely cellular, short, interlacing bundles and fascicles with vague areas of palisading nuclei (Antoni type A, Figures 6-8). The remaining 40 percent was organized in loose streams and sheets where the cells were interspersed with pale eosinophilic wispy material (Antoni type B, Figure 9). The cells contained a scant amount of pale, fibrillar cytoplasm, with variably distinct cellular borders. Central nuclei were oval to fusiform with coarsely to finely stippled chromatin. The cells displayed mild anisokaryosis and anisocytosis and mitotic figures were noted rarely. The mass extended to the surgical margins. The histologic diagnosis was a low grade nerve sheath tumor.
Discussion
Nerve sheath tumors (NST, also called peripheral nerve sheath tumors or PNST*) have traditionally been thought of as uncommon cutaneous tumors in cats.1–3 NST can occur within the skin or subcutaneous tissues and, in cats, are most commonly found on the head and neck, followed by the limbs.3,4 In one study, cutaneous NST accounted for 7.7% (26/337 tumors) of feline cutaneous and subcutaneous tumors over a 12 year period.1 Therefore, inclusion of NST in the differential diagnoses for neoplasms of spindle-shaped cells in the skin and subcutaneous tissue of cats is warranted.
Cutaneous NST arise from small nerves in the skin and subcutaneous tissues where the cell of origin may be a Schwann cell (schwannoma), perineurial cell (perineuroma), or a mixture of Schwann cells, perineurial cells, and fibroblasts (neurofibroma).1 Immunohistochemical (IHC) staining is needed to determine the cell of origin.2,4,5 Because the biologic behavior of these tumors is similar and immunostaining in these tumors is often overlapping or non-specific, a diagnosis using the umbrella term of “nerve sheath tumor” is typically made without further ancillary IHC testing to more conclusively identify the cell of origin.2
Nerve sheath tumors are composed of spindled cells with wavy cytoplasm arranged in variably compact areas with occasional nuclear palisading or whorls.1,6 Some tumors will have densely cellular areas (Antoni A) that include nuclear palisading and are juxtaposed with more loosely cellular areas (Antoni B). Human schwannomas have a classic appearance including Antoni A configurations with Verocay bodies.6 This classic Antoni A pattern is rarely identified in veterinary species.2 The absence of well-defined Antoni A and B areas, nuclear palisading, or Verocay bodies does not rule out a diagnosis of NST. NST from feline patients commonly have a plexiform growth pattern.2
In dogs, NST are included within the broader category of soft tissue mesenchymal tumors or soft tissue sarcomas (STS). The term STS refers to a group of neoplasms that share similar biologic features despite being thought to originate from different elements of soft tissue.2 For example fibromas, fibrosarcomas, myxomas, and myxosarcomas are all believed to arise from fibroblasts, while myopericytomas, hemangiopericytomas, and angioleiomyomas originate from cells within vascular walls. Most notably STS tumors as a group tend to be locally expansile or infiltrative, but have a low metastatic rate.2,3,7 Perivascular wall tumors (PWT) have a very similar morphologic appearance to that of NST and are also included in the STS group in dogs.2 While histogenesis of PWT remains controversial and some pathologists do not use this morphologic diagnosis, these tumors may originate from pericytes in the vascular wall.7 The myopericytoma variant of PWT is considered the principal differential diagnosis for NST in the dog. However, due to similar biologic behavior, this distinction is not thought to be clinically relevant.2 NST of dogs will typically stain positively for neuron-specific enolase (NSE), S100, and neurofilaments, with variable staining reported for nerve growth factor receptor (NGFR), myoglobin, and glial fibrillary acidic protein (GFAP).2,7 PWT typically stain positive for calponin and pan-actin, with variable staining for smooth muscle actin, and typically are negative for S100, NSE, GFAP, and myoglobin.2,7 In cats, positive staining for NST has been reported with vimentin, S-100, NSE, GFAP, and laminin with variable expression of smooth muscle actin and the neuronal marker, PGP 9.5. There is variable expression of the proliferation marker, Ki67.1,4
In dogs, histologic grading of STS has been shown to predict local recurrence and development of metastasis.7 The currently accepted rubric is based on evaluation of cellular differentiation, mitotic activity, percent necrosis, and margin of excision.7 Feline NST are also divided into benign and malignant tumors by many authors, however the criteria evaluated differ between studies and do not always include tissue invasion, surgical margins, or follow-up.1,4 Thus, there is currently no accepted histologic grading scheme for prediction of biologic behavior of NST in cats. Various features evaluated in these studies include cellularity, cellular polymorphism, tissue infiltration or invasion, mitotic index, and necrosis.1,4 In general, a higher mitotic rate and increased cellular pleomorphism are associated with more malignant behavior.
Surgical excision is the preferred treatment for NST and the likelihood of recurrence is dependent on: 1) Adequacy of excision, 2) Mitotic activity, and 3) Histologic grade.2,3,7 In one study of 53 cats with NST, no distant metastases were reported.4 However, the authors did report local tumor recurrence in 20% (9/45) of cats for whom follow-up was available. There is an individual case report of an extremely aggressive, locally extensive malignant form.8 Because the mass in this case was incompletely excised, monitoring for local recurrence was recommended. At the time of writing (approximately 7 months after surgical removal of the mass), the primary care veterinarian reported that the patient was doing well, with no regrowth.
* Since all nerves are peripheral, it has been argued that the tumors should simply be referred to as “nerve sheath tumors.”9
References
- Mandara, M. T., Fabriani, E., Pavone, S. & Pumarola, M. Feline cutaneous nerve sheath tumours: Histological features and immunohistochemical evaluations. Res. Vet. Sci. 95, 548–555 (2013).
- Hendrick, M. J. Mesenchymal Tumors of the Skin and Soft Tissues. in Tumors in Domestic Animals (ed. Meuten, D. J.) 142–175 (John Wiley & Sons, Inc., 2016). doi:10.1002/9781119181200.ch5.
- TL Gross. Neural and Perineural Tumors. in Skin diseases of the dog and cat: clinical and histopathologic diagnosis. (ed. Gross, T.) 786–796 (Blackwell Science, 2005).
- Schulman, F. Y., Johnson, T. O., Facemire, P. R. & Fanburg-Smith, J. C. Feline Peripheral Nerve Sheath Tumors: Histologic, Immunohistochemical, and Clinicopathologic Correlation (59 Tumors in 53 Cats). Vet. Pathol. 46, 1166–1180 (2009).
- Chijiwa, K., Uchida, K. & Tateyama, S. Immunohistochemical Evaluation of Canine Peripheral Nerve Sheath Tumors and Other Soft Tissue Sarcomas. Vet. Pathol. 41, 307–318 (2004).
- Wippold, F. J., Lubner, M., Perrin, R. J., Lämmle, M. & Perry, A. Neuropathology for the Neuroradiologist: Antoni A and Antoni B Tissue Patterns. Am. J. Neuroradiol. 28, 1633–1638 (2007).
- Dennis, M. M. et al. Prognostic Factors for Cutaneous and Subcutaneous Soft Tissue Sarcomas in Dogs. Vet. Pathol. 48, 73–84 (2011).
- Stoll, A. L., Suárez-Bonnet, A., Summers, B. A. & Priestnall, S. L. Malignant Cutaneous Peripheral Nerve Sheath Tumour with Rhabdomyosarcomatous Differentiation (Triton Tumour) in a Domestic Cat. J. Comp. Pathol. 165, 1–5 (2018).
- de Lahunta, A. Feline Nerve Sheath Tumors Versus Feline Peripheral Nerve Sheath Tumors. Vet. Pathol. 47, 758–758 (2010).
Authored by: Drs. C. Haak (resident) and K. Saunders; edited by T Stokol.