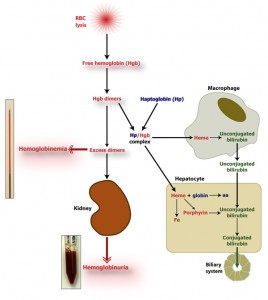
The hemoglobin dimers that remain in circulation are oxidized to methemoglobin, which disassociates into a free heme and globin chains. The oxidized free heme (met-heme) binds to hemopexin (a β-globulin, Hpx) and the met-heme and hemopexin complex (met-heme/Hpx) is taken up by a receptor on hepatocytes and macrophages within the spleen, liver and bone marrow (only hepatocyte uptake is illustrated in the image above). Similarly, the hemoglobin/haptoglobin complex is taken up by hepatocytes and macrophages (to a lesser extent). Within these cells, the hemoglobin disassociates into heme and globin chains. The globins are broken down to amino acids, which are then used for protein synthesis. The heme is oxidized by heme oxygenase forming biliverdin and releasing iron. The iron can be transferred to apotransferrin (the iron transport protein) in plasma or can be stored within cells as ferritin (i.e. the iron is bound to the storage protein, apoferritin). The remaining porphyrin ring (biliverdin) is degraded to unconjugated bilirubin by biliverdin reductase. If the hemoglobin/haptoglobin complex is internalized by macrophages, the unconjugated bilirubin is released into the plasma, where it binds to albumin (to render it water-soluble) and is taken up by hepatocytes through the haptoglobin receptor.