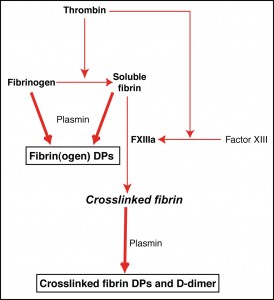
Thrombin is responsible for fibrin formation and for fibrin cross linking. Fibrinogen consists of two pairs of three polypeptide chains, called α, β, and γ chains. These form a dimer by covalently joining at one end, with the central region being called the E domain and the two terminal D regions, called the D-domains.
A soluble fibrin monomer is produced when thrombin cleaves fibrinogen (from the E region of the α chains) to yield fibrinopeptide A (and to a lesser extent, thrombin also cleaves the E region of the β chains to yield fibrinopeptide B). This cleavage allows fibrin to spontaneously associate to form a polymer, through longitudinal covalent associations of the D-domains (terminal ends of the fibrinogen α, β, and γ chains). Lateral associations between fibrin polymers also form, creating a soluble fibrin network. Thrombin also activates the crosslinking enzyme, factor XIII. Once activated, FXIIIa creates crosslinks between the γ chains of the D-domain between adjacent fibrin monomers within a polymer (γ-γ links), stabilizing the polymer and creating a new antigen (neo-epitope), called D-dimer, in the process. Factor XIIIa also creates crosslinks between the α chains of fibrin (α-α links), between adjacent polymers thus stabilizing the network.
Traditional fibrin(ogen) degradation products (X, Y, D and E), which are measured in serum or plasma, are released when plasmin cleaves fibrinogen or soluble fibrin (which differ from each other by fibrinopeptide A and B). In contrast, crosslinked degradation products of variable size are released when plasmin cleaves crosslinked fibrin; the smallest crosslinked product is D-dimer, which is measured in plasma. Since crosslinking requires thrombin to activate factor XIII and create the D-dimer neo-epitope, D-dimer is specific for crosslinked fibrinolysis. In contrast, FDPs can be yielded by plasmin-mediated cleavage of fibrinogen without thrombin being present.