A series of consensus statements on transfusion reactions established by Association of Veterinary Hematology and Transfusion Medicine has been published, including definitions and types of reactions (Davidow et al 2021a), preventing and monitoring transfused patients for reactions (Davidow et al 2021b), methods for detecting certain transfusion reactions and recommendations for treatment (Odenuyo et al 2021).
Transfusion reactions can occur acutely with transfusion or within 24 hours of transfusion (called delayed) are usually separated into two mechanistic categories: immunological and non-immunological. The most common reactions to transfusions are fever, vomiting and facial edema. These are generally mild. More severe reactions, including intravascular hemolysis, shock and dyspnea, are seen far less frequently. In a study of 133 cats administered 246 transfusions, adverse reactions were seen in 6.3% of the 191 whole blood or packed red cell transfusions. No reactions were seen with 55 fresh frozen plasma transfusions. The reactions consisted of (in decreasing frequency) transient pyrexia, delayed hemolysis, transfusion-associated circulatory overload (TACO), facial edema, acute hemolysis and death. In a study of 131 dogs administered 163 packed red blood cell transfusions, 13% of the dogs suffered acute (vomiting, intravascular hemolysis, icterus) or delayed (rapid decrease in post-transfusion hematocrit) hemolytic transfusion reactions. Similarly, in a retrospective study of 953 transfusions (packed red blood cells, plasma, plasma plus packed red blood cells and whole blood) to 558 dogs, there was a 15% incidence of transfusion reactions, most of which occurred in response to transfusions of packed red blood cells. The most common type of reaction was fever, followed by vomiting (mostly in dogs given packed red blood cells). Allergic reactions were seen in dogs given packed red blood cells or plasma and may have been alleviated by prior antihistamine medication (seen only in 1 premedicated dog versus 12 non-premedicated dogs). Four dogs (10%) suffered hemolytic reactions, including intravascular hemolysis and icterus (within 8 hours of transfusion) even though they had not received blood transfusions before. The reasons for the hemolysis were not ascertained but one dog had IMHA. Some dogs died as a consequence of the reactions (Bruce et al 2015).
Transfusion reactions can be minimized (and even prevented in some instances) by crossmatching or typing animals prior to transfusion and by paying strict attention to transfusion procedures, e.g. maintaining strict aseptic techniques, infusing products slowly, not using out-of-date or discolored products, closely observing the animal during the transfusion procedure (especially in the first 15 to 30 minutes) and not forcing the transfused product through small gauge catheters.
In general, treatment of transfusion reactions is symptomatic and logical. First and foremost, if a transfusion reaction develops, the transfusion should be stopped, although this depends on the severity and type of reaction. If sepsis is suspected, the product should be sent for bacteriological culture (cytologic assessment of the transfusion product may also identify bacteria if in sufficient numbers). The animal should be treated symptomatically, e.g. if showing signs of an anaphylactic reaction, epinephrine can be administered whereas if there is an allergic non-anaphylactic reaction, antihistamines can be given (Odenuyo et al 2021). Currently, it is not recommended to administer pre-transfusion corticosteroids and/or antihistamines to try and prevent an allergic-type transfusion reaction. Allergic reactions are generally mild and can be minimized by sby not mixing multiple bags from different donors. Many clinicians also slow the rate of the infusion, although there is no evidence supporting this practice (Odenuyo et al 2021). If an animal is known to have had previous hypersensitivity reactions, then pre-medication with anti-allergic drugs is indicated.
Immunologic reactions
Immunological transfusion reactions can be hemolytic or non-hemolytic in nature. Both types can be separated into acute (those occurring immediately after transfusion) and delayed reactions. The worst type of reaction is the acute hemolytic reaction, which can result in death of the animal.
Hemolytic immunological transfusion reactions
- Acute hemolytic transfusion reaction: An acute hemolytic transfusion reaction (AHTR) is due to a blood type incompatibility (i.e. there are antibodies in recipient serum against donor red blood cell antigens) and is a class II (antigen-antibody) hypersensitivity reaction. It occurs during or within 24 hours of a transfusion (Davidow et al 2021a). A disastrous acute hemolytic crisis is what is often pictured as the typical transfusion reaction, however, in reality, these reactions are quite uncommon, particularly in dogs and horses. These species lack naturally occurring antibodies to major red blood cell antigens and severe acute reactions are due to acquired antibodies, such as secondary to prior transfusions or pregnancy. The antigens involved in acute hemolytic reactions are DEA 1.1 and 1.2 in the dog and Aa and Qa in the horse (there has been one report of an acute hemolytic reaction in a DEA 1.1-negative, 1.2-positive Whippet administered numerous bags of DEA 1.1-negative blood, suggesting a reaction to a common blood group antigen (none of the known blood types) that was absent in the dog as well as a related sibling). In cats, an acute hemolytic reaction can occur on the first transfusion as naturally occuriring antibodies are found in this species. The most severe reaction occurs in type B cats administered type A or type AB blood.
The severity and timing of an acute immune-mediated hemolytic reaction depends on the type of antibody involved (IgM or IgG), the temperature at which they bind to the antigen and how efficiently they fix complement. Hemolysis can be extravascular or intravascular, with consequences of intravascular hemolysis being more serious. With intravascular hemolysis, there is systemic activation of hemostasis (thus initiating DIC) and production of systemic hypotension (due to the release of vasoactive substances such as C3a, C5a, bradykinin), shock (due to cytokine release) and renal failure (from ischemic necrosis due to hypoxia, DIC and renal vasoconstriction).
Clinical signs of a severe acute hemolytic reaction are, unfortunately, non-specific and include tachycardia, dyspnea, collapse, hypotension, salivation, tremors, convulsions, weakness, vomiting and pyrexia. Acute intravascular hemolysis produces hemoglobinemia (as shown in the image on the left) and hemoglobinuria, which can occur with minutes of transfusion. Extravascular hemolysis is due to destruction of antibody-coated donor erythrocytes by the macrophages in the spleen and liver, leading to hyperbilirubinemia and bilirubinuria. Clinical signs are generally milder than those produced by intravascular hemolysis.
If an acute hemolytic reaction develops, the transfusion should be immediately stopped and supportive therapy instituted. This includes administration of intravenous fluids to combat hypotension and shock and maintain renal perfusion. Some authors also advocate the use of corticosteroids with or without low doses of dopamine. Treatment of DIC is also indicated (and controversial in itself). In general, acute hemolytic transfusion reactions can be prevented by crossmatching or using only matched typed blood. Remember, that all cats should be typed or crossmatched before their first blood transfusion (although depending on the breed, the likelihood of having a type B cat can be quite low). - Delayed hemolytic reaction: Delayed hemolytic transfusion reactions are a result of extravascular hemolysis occurring after 24 hours to up to 28 days after transfusion (Davidow et al 2021). This reaction may occur in dogs that are administered incompatible blood on the first transfusion. It takes about 4- to 7 days to get an antibody response to the foreign red blood cells (called delayed serologic transfusion reaction [Davidow et al 2021a]). Thus after the production of antibody, there is accelerated removal of the donor erythrocytes and the transfusion does not last as long as it should. This also occurs in dogs when they are transfused with blood types to which they possess natural occurring antibodies (against DEA 3, 5 and 7) or have been sensitized to an antigen (that elicits a delayed hemolytic reaction, rather than the acute hemolytic reaction seen with antibodies against DEA 1.1 and 1.2) via previous incompatible transfusions, once an anamnestic response has been elicited.
Delayed hemolytic reactions are usually mild and may not be recognized. This type of reaction should be suspected in a patient in which the post-transfusion hematocrit rapidly declines or does not stay elevated for as long as it was expected to, or that develops hyperbilirubinemia and/or bilirubinuria. The most common signs are fever, anorexia and icterus. A positive direct Coombs test will also be seen. Specific treatment for this type of reaction is generally not required. This type of reaction can be minimized by using matched typed or crossmatched blood (remember that the crossmatching procedure will not detect low titers of antibodies, therefore these reactions cannot be totally prevented). All dogs should be crossmatched after a second blood transfusion and some authors have advocated for crossmatching before the first transfusion (Bruce et al 2015). Note that typing does not supplant crossmatching, because there are as yet unknown antigens that could result in transfusion reactions (particularly delayed) and we cannot type animals for all known antigens (we keep discovering more). - Neonatal isoerythrolysis: This occurs when a female animal of one blood type is mated to a male of another. Neonates that inherit the blood group of the sire develop hemolytic anemia when they ingest colostrum containing antibodies against their and the sire’s erythrocytes. This has been reported in cats(Silvestre-Ferreira and Pastor 2010), dogs, cattle (as a consequence of Anaplasma vaccination [Searl 1980]), pigs and horses (Boyle et al 2005, Polkes et al 2008). In cats, it occurs with type B queens mated to A or AB toms. In this situation, all kittens bearing the type A antigen will suffer a hemolytic anemia at birth after ingestion of colostrum containing naturally occurring anti-type A antibodies (Silvestre-Ferreira and Pastor 2010). In horses and dogs, this occurs in dams that have been previously sensitized to blood group antigens (types Aa and Qa especially in the horse, although other blood groups can also be the culprit, and DEA 1.1 and 1.2 in the dog). Neonatal isoerythrolysis has also been reported in mule foals, because of a unique donkey-specific RBC antigen, which mares lack (McClure et al 1994, Traub-Dargatz et al 1995). However, not all mule foals develop neonatal isoerythrolysis, so it is unclear why this syndrome is uncommon and only occurs with some mares. Hemolytic anemia is due to extravascular hemolysis, but some animals may suffer from intravascular hemolysis (resulting in hemoglobinemia and hemoglobinuria). Clinical signs in affected neonates include weakness, failure to thrive, and death (fading kitten/puppy/foal syndrome). Affected kittens can also suffer from tail tip necrosis (presumably from agglutination of RBCs which block off vessels in the tail tip) (Bride and Littlewood 1998). A Coomb’s test on the neonate will be positive and a crossmatch between the dam’s serum and neonate’s erythrocytes will reveal the incompatibility (for more information, see mare-foal incompatibility). Neonatal immune-mediated thrombocytopenia has also been reported in foals (equine and mule) and piglets (Traub-Dargatz et al 1995, Buechner-Maxwell et al 1997, Ramirez et al 1999, Forster 2007).
There have been two case series of neonatal isoerythrolysis in foals. In the first series of 18 foals, common affected breeds were mules, Paints, standardbred and thoroughbred, with no cases in Arabs, miniature horses or draft breeds. Foals presented at a mean of 71 hours of birth (range 8 hours to 10 days). Around a third were febrile, tachypneic with around 20% being tachycardic. Many foals (11/18) had icterus, some also had murmurs. The mule foal had concurrent thrombocytopenia. Hemoglobinuria indicating intravascular hemolysis was seen in 3/5 foals in which urine was collected. The hematocrit in affected foals was a mean of 17% (range, 9-38%), with 50% being >15%. The foals with the normal hematocrits had failure of passive transfer of immunity, suggesting they did not get a high dose of the alloantibodies in colostrum. The degree of anemia did not correlate to outcome. Some foals had an inflammatory leukogram and high fibrinogen. Three foals were thrombocytopenic, including the mule foal. Common findings on a hemogram were high total bilirubin, mostly unconjugated with 5/14 tested foals having increases in unconjugated bilirubin, supporting concurrent cholestasis. Two foals, in which urine was collected, had bilirubinuria. High SDH, indicating acute liver injury (probably as a consequence of hypoxia from severe anemia) was seen in the majority of foals. Liver biopsy in some foals that dies showed evidence of hepatic necrosis, multifocal neutrophilic hepatitis with lobular collapse, bile duct hyperplasia and early fibroplasia, hemosiderosis (excess iron turnover from hemolysis) and Kuppfer cell hyperplasia. All tested foals (n=5) had a lactic acidosis (L-lactate from anaerobic metabolism). The diagnosis was accomplished in 8/9 foals on the basis of a positive Coomb’s test. Other foals were diagnosed with a positive crossmatch or anti-erythrocyte antigen testing. Offending antigens were Aa, Qa, Pa, Qb, Dg, Qa+Qb+Qc. The lowest hematocrit was seen in foals with RBCs that were positive for Dg (not previously implicated with isoerythrolysis), Aa, Qa and the combination of Qs. Foals were treated with whole blood transfusions (crossmatched donor, washed dam RBCs), oxyglobin and supplemental oxygen. Most of the foals survived (83%), with 3 foals being euthanized due to sepsis, hepatic injury and neurologic disease (Boyle et al 2005). The second study of 75 foals largely focused on prognostic factors and found that foals, which were mostly thoroughbreds, died of liver injury or failure, complications due to sepsis and kernicterus (deposition of unconjugated bilirubin in brain). The liver failure was attributed to hypoxic injury and hemosiderosis from repeated transfusions). The survival rate was 75% (Polkes et al 2008).
Non-hemolytic immunological transfusion reactions
- Febrile non-hemolytic transfusion reaction (FNHTR): This is defined as an acute increase in body temperature >1°C within 4 hours of the end of a transfusion and a temperature of >39°C or 102.5°F that cannot be explained by other conditions, including other transfusion reactions. It is attributed to an immunologic reaction to donor leukocytes or platelets or non-immunologic reaction from pro-inflammatory cytokines in the transfused product (Davidow et al 2021a). It is most common type of transfusion reaction observed with whole blood. In human patients, this reaction has only been observed with whole blood, red cell or platelet concentrates, and fresh plasma. It has not been observed with frozen plasma products (FFP or cryoprecipitate). The reaction is suspected when there is a temperature increase of at least 1°C with no other cause for the elevation. This reaction has been attributed to the presence, in the recipient’s plasma, of antibodies reactive against antigens on donor leukocytes or platelets or to cytokines (TNF, IL-1, IL-6), that are actively produced by donor leukocytes and accumulate in the transfusion product with storage.
These reactions can occur on the first transfusion, with fever being noted within the first 30 minutes (and can persist for up to 20 hours). They have been reported in horses, along with muscle fasciculations, tachypnea, arrhythmia and sweating in adult horses given allogeneic crossmatch incompatible transfusions (first transfusion) (Tomlinson et al 2015). Vomiting and tremors may also be seen.
In severe cases, the transfusion should be stopped. In milder cases, the transfusion rate can be slowed or stopped then restarted at a slower rate. Febrile reactions can be minimized by leukoreduction of the product prior to transfusion. This can be accomplished by the use of special filters in transfusion lines. One such filter has been evaluated in dogs and effectively reduced the number of transfused donor leukocytes in whole blood transfusions by 87 to 99%, without affecting post-transfusion red cell viability. - Acute hypersensitivity: Acute hypersensitivity reactions are usually due to anaphylactic (allergic or type I hypersensitivity) reactions and can begin during the reaction or up to 4 hours after transfusion. This is mediated by IgE antibodies which activate mast cells, which then release inflammatory and vasoactive mediators, that produce hypotension and increased vascular permeability. Dogs can vomit, have urticaria or facial edema, suffer from diarrhea or hemoabdomen, which is seen in anaphylactic reactions of various causes. Cats usually have respiratory signs, e.g. bronchoconstriction, edema, but can also vomit and have facial edema or angioedema. Incidence varies from 0-7% (Davidow et al 2021a). Another type of reaction is an anaphylactoid reaction, which is not mediated by IgE. The exact mechanism of this reaction is uncertain, but it is likely due to cytokines or other products which activate the same inflammatory and vasoactive mediators likely via IgG binding to Fc receptors on macrophages. Allergens in the donor blood include antibiotics or chemicals used in blood preparation, albumin and C4. Rarely, transfused IgE antibodies from the donor can initiate the reaction. This type of reaction is frequently seen with infusion of plasma (fresh, fresh frozen or frozen) and cryosupernatant. It has not been documented with infusion of cryoprecipitate.
This type of reaction can occur on the first transfusion and has been seen in horses given cross-match incompatible blood (Tomlinson et al 2015). Clinical signs occur rapidly after transfusion (within 1 to 45 minutes) and range from minor skin reactions (this is the most common reaction, consisting of pruritis, facial edema, wheals, urticaria) to more severe allergic reactions, including hypotensive shock, bronchoconstriction and cardiopulmonary arrest.
Treatment consists of stopping the transfusion. For mild reactions, administration of antihistamines will rapidly alleviate the clinical signs. More severe signs require intensive shock therapy, including administration of epinephrine. Corticosteroids are no longer recommended to treat this type of reaction (Odenuyo et al 2021). This type of reaction can be minimized by pretreatment with antihistamines (in predisposed animals), possibly by slow infusion rates, not pooling products from multiple donors, and being careful with selection of components (i.e. use cryoprecipitate instead of plasma when indicated). - Delayed transfusion-related thrombocytopenia: Post-transfusion purpura is a rare condition in human patients characterized by a severe thrombocytopenia 1 to 2 weeks after blood or blood product transfusion. It is believed to be due to the development of platelet-specific antibodies that destroy both the transfused and the patient’s own platelets. This has been reported in a dog with hemophilia A. The dog developed a severe thrombocytopenia (platelet count < 10,000/µL), on 2 occasions after being transfused (the first occurred 8 days after a second blood transfusion; the second, 5 days after cryoprecipitate infusions). On both occasions, the platelet counts normalized within 4 to 6 days of corticosteroid therapy (but the dog may have recovered spontaneously without treatment). No specific treatment is required.
- Neonatal alloimmune thrombocytopenia: This occurs because of differences in platelet antigen expression in sires and dams, similar to neonatal isoerythrolysis. This has been recognized in pigs, mules and a Quarterhorse foal (Buechner-Maxwell et al 1997, Ramirez et al 1999, Forster 2007). In pigs, agglutinating antibodies against sire and piglet platelets have been documented in the dam. The piglets are generally born healthy, then develop thrombocytopenia after 5 to 9 days, with the nadir occurring at 10 to 13 days. Thrombocytopenia is due to increased platelet destruction and decreased platelet production. Clinical signs of cutaneous hemorrhage are seen. Death may result at 2 to 3 weeks of age. Surviving piglets appear clinically and hematologically normal by 16 weeks of age. Severe thrombocytopenia was discovered in a 1 day old Quarterhorse foal presenting for weakness and failure to suckle. The dam’s serum contained antibodies that bound to the foal’s and a full brother’s platelets (Buechner-Maxwell et al 1997). We have also similar cases in foals (equine and mule) at Cornel University.
- Neonatal alloimmune neutropenia: This has been reported in a foal with concurrent neonatal isoerythrolysis (Wong et al 2012).
- Transfusion-related acute lung injury: This manifests as acute respiratory distress syndrome within several 6 hours of the end of or during a transfusion in patients with no prior lung disease. Clinical signs include dyspnea, tachypnea, tachycardia, fever and non-cardiogenic pulmonary edema. Animals are hypoxemic. The cause of TRALI is unknown, but it is attributed to an immunologic reaction to antigen-antibody complexes (Davidow et al 2021a). Due to difficulties in documenting this syndrome, the incidence in dogs and cats is relatively unknown.
- Immunosuppression: Donor lymphocytes in the transfused whole blood or blood product are believed to suppress immunity in the recipient. In extreme cases in human patients (especially with multiple transfusions), transfusion-associated graft versus host disease can result (this has not been reported with frozen plasma products). Immunosuppression or graft versus host disease can be reduced by leukoreduction or destruction of immunocompetent donor lymphocytes (such as by radiation). This type of transfusion reaction has not been recognized in animals.
- Transfusion associated graft versus host disease. This is where donor lymphocytes cause an immune-mediated reaction against recipient cells and is often fatal, but fortunately, rare in humans. It has been experimentally induced in dogs with repeated transfusions of viable leukocytes (Davidow et al 2021a).
Non-immunologic reactions
These consist of the following reactions, which are not mediated through immune mechanisms:
- Transfusion-associated circulatory overload (TACO): This is defined as an acute onset of dyspnea associated with increased blood volume and pulmonary edema with a reported incidence of 3-5% in dogs and cats (Davidow et al 2021a). It can occur in young animals or patients with cardiovascular disease. Normovolemic adult patients can also develop circulatory overload if transfused with large amounts of blood. The clinical signs are cardiovascular in nature (coughing, tachypnea, dyspnea and tachycardia). In severe cases, pulmonary edema, congestive heart failure, and vomiting may occur. This reaction can be minimized by using appropriate component therapy and slow infusion rates (especially in small or compromised patients). If the reaction occurs, the transfusion should be stopped and furosemide given. Measurement of brain natriuretic peptide may be helpful in diagnosis of this syndrome (Davidow et al 2021a, Odenuyo et al 2021), but is not usually available as a point-of-care test, where it would be most helpful.
- Transfusion-associated dyspnea: This is another category defined in humans, which encompasses cases of dyspnea that cannot be attributed to TACO or TRALI and occurs within 24 hours after the end of a transfusion (Davidow et al 2021a). This has an incidence of 2-7% in studies of dogs and cats (Davidow et al 2021a), although cases of TRALI and TACO likely fall into the latter because extensive testing for the cause of dyspnea is not always done.
- Hemolysis: Non-immunological hemolysis of erythrocytes occurs secondary to bacterial contamination, inappropriate administration, mishandling and use of out-dated products. Mechanical hemolysis can occur with infusion through small needles or catheters, plugged lines or rapid infusion. Some peristaltic pumps can also cause mechanical hemolysis. Overheating (> 37°C) or freezing of erythrocytes will also cause hemolysis as will administration of red cells with hypotonic solutions (such as 5% dextrose in water). Non-immunological hemolysis can be prevented somewhat by paying close attention to handling and administration of whole blood and blood products, however prolonged storage of blood products may result in hemolysis regardless. A study of 180 packed RBC units showed minimal hemolysis after collection, with hemolysis occurring with increased storage time in just over half of the units by 6 weeks of storage refrigerated. Such hemolyzed products may not meet blood bank specifications for transfusion (Ferreira et al 2018).
- Bacterial contamination or transfusion-transmitted infection: Blood is an excellent culture medium and bacteria that can proliferate at cold temperatures, such as Pseudomonas and Serratia species, can contaminate blood units and lead to transfusion reactions (Stefanetti et al 2016), which can be acute or delayed. A study of 49 blood units, showed a low rate of bacterial contamination with gastrointestinal organisms (Enterococcus and Escherischia coli) (Miglio et al 2016). Infusion of gram-negative bacteria (dead or alive) will result in endotoxic shock, with clinical signs of fever, hypotension, hemolysis, vomiting, diarrhea and DIC. Contaminated blood is usually dark or discolored and may contain bubbles, particulate material or clots. If a reaction occurs, the transfusion should be ceased and the bag cultured and gram-stained. Supportive care is then instituted, however the prognosis is poor. Contamination can be kept to a minimum by the use of aseptic techniques (for blood transfusion collection from the donor and for administration) and by completing a transfusion over 3 to 4 hours.
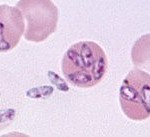
Other infectious agents, including heartworm microfilaria, spirochetes (Borrelia), rickettsia (Ehrlichia), Babesia and hemotrophic Mycoplasma (there is a high incidence of seropositivity for Babesia in retired racing Greyhounds, a favored breed for blood donation; the above image is Babesia discovered in a splenectomized Greyhound; in the past splenectomy or immunosuppressive doses of corticosteroids could result in recrudescence of disease in carriers) and viruses (FIV and FeLV) can be transmittted from the donor to the recipient. All donors should be negative on serology or PCR for infectious organisms. PCR reactions and serologic testing may be negative in carrier animals.
- Hypotensive transfusion reactions: This occurs when blood products contain vasoactive compounds and results in hypotension, acutely after starting an effusion. This has not been reported in dogs and cats (Davidow et al 2021a).
- Hypocalcemia: An acute hypercalcemia can result from citrate toxicity, which chelates calcium. In the absence of liver disease (which metabolizes citrate to bicarbonate), clinically significant hypocalcemia is unlikely to occur. In animals without liver disease, rapid transfusion of large amounts of plasma products may overwhelm the liver’s ability to remove the citrate. In one study in dogs, citrated blood or fresh frozen plasma was associated with ionized hypocalcemia in all cases, especially those transfusions associated with > 40% blood replacement (the number of dogs showing clinical signs of hypocalcemia was not stated). Clinical signs of citrate toxicity include tremors, tetany, vomiting and ventricular arrythmias. Hypocalcemia is rapidly reversible by slowing or temporarily ceasing the transfusion. Calcium infusions can be used in severe cases.
- Coagulopathy: Massive transfusions of stored blood (which lacks platelets and Factors V, VIII and IX) may result in a coagulopathy. Furthermore, diluting packed red cells in solutions containing calcium may cause in vitro clotting. This can be avoided by diluting packed red cells in sterile isotonic (0.9%) saline.
- Hyperammonia and acidosis: Erythrocytes are rich in ammonia and ammonia levels increase in stored whole blood. This can cause ammonia toxicity with associated neurologic signs, although this has not been reported in veterinary medicine (Davidow et al 2021a). The pH of stored blood decreases with storage due to anaerobic metabolism of glucose, with lactic and pyruvic acid production. Massive transfusions may lead to post-transfusional acidosis, but this is exceedingly rare as the liver converts these acids into bicarbonate (thus offsetting the acidosis).
- Hypothermia: Significant hypothermia can result if transfusing whole blood which has not been warmed. This will especially occur in small or young animals.
- Embolism: Microaggregates of platelets, leukocytes and fibrin, which form in whole blood stored for more than 7 days, may be associated with pulmonary thromboembolism (although this is controversial). These range in size from 20 to 120 μm and may not removed by standard filters (approximately 150 μm). The use of small (20 to 40 μm) filters is not recommended, because it may slow down the transfusion needlessly.
- Hemosiderosis: Iron overload from massive transfusions of whole blood or blood products is a rare complication.
