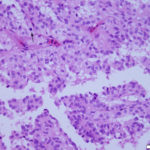
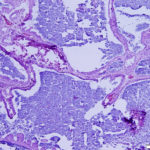
Interpretation
Epithelial hyperplasia or well-differentiated neoplasia with histiocytic (granulomatous) inflammation
Explanation
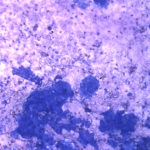
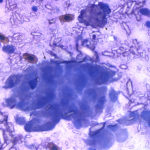
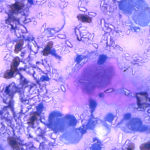
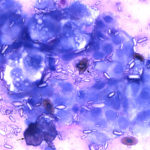
The aspirate consisted of many crystals, with several circular to trabecular clusters and a few flat sheets of medium to large round to polygonal to angular epithelial cells, with low numbers of macrophages and a few erythrocytes (Figure 2a and 3a; Question 1). The epithelial cells had central to paracentral nuclei with lacy chromatin and single nucleoli (Figure 4). They had moderate amounts of light purple cytoplasm and displayed mild to moderate anisocytosis and anisokaryosis. Some of the cells had areas of rarefied cytoplasm or contained moderate numbers of discrete to coalescing cytoplasmic vacuoles, usually at the periphery of the cell (Figure 5). A small number of bright pink smooth thick strands of extracellular matrix were seen between some of the epithelial clusters (supportive stroma, presumptive). The crystals were variably sized (ranging from 2-20 um wide and 2-40 um in length), clear to light pink, rectangular and usually parallel-sided, with ovoid or pointed ends. Most were flat and one dimensional but several appeared to have daughter or budding crystals. Crystals were abundant extracellularly but were also seen phagocytized within macrophages (Figure 3a and 5). The cytologic diagnosis was epithelial hyperplasia or well-differentiated neoplasia with histiocytic inflammation (Question 2).
Additional tests
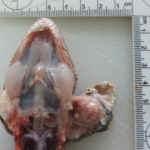
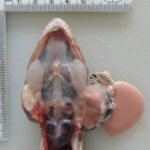
The aspirated fluid was submitted to the Texas urolithiasis laboratory for analysis of the crystals, which came back as calcium carbonate (although the crystals did vaguely resemble some variants of calcium oxalate crystals seen in equine urine [see urine crystal album]). Considering the rapid progression of the mass and potential for bone invasion, the decision was made to humanely euthanize the gecko. On post-mortem examination, two masses were found within the left endolymphatic sac (Figures 6 and 7). The right endolymphatic sac was also enlarged but contained only white opaque fluid with no discernible mass lesions.
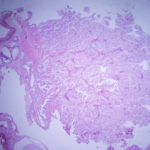
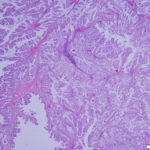
On histologic examination of harvested post-mortem tissues, the masses in the left endolymphatic sac consisted of papilliferous projections of columnar epithelial cells supported by a thin fibrovascular stroma (Figures 8 and 9). In some areas, the cells formed thicker lobules or islands with evidence of disorganization (Figure 10). The cells displayed moderate anisocytosis and anisokaryosis and several mitotic figures (ranging from 0-4/40x high power field) were seen (Figure 10). A few inflammatory cells (lymphocytes, granulocytes and macrophages with intracellular pigment) were identified within the supporting stroma, however more macrophages, containing phagocytized crystals, and other inflammatory cells were seen in the adjacent hyperplastic sac lining and within the lumen of the sac, along with extravasated erythrocytes. In contrast, the lining of the right endolymphatic sac consisted of relatively flat multiloculated projections of uniform columnar epithelium with abundant intraluminal mineral (Figure 11). Multifocal regions of sterile granulomatous and granulocytic inflammation were observed in the right endolymphatic sac wall. The final histologic diagnosis was an endolymphatic carcinoma (in situ, low grade) with adjacent epithelial hyperplasia and chronic (granulomatous) inflammation in the non-neoplastic lining. The right endolymphatic sac had multifocal granulomatous and granulocytic inflammation. Other findings on histologic evaluation included interstitial fibrosis in both kidneys, remodeling of cranial vertebrae and diffuse hepatic atrophy.
Discussion
The endolymphatic sac originates from the neuroectoderm and is a terminal dilation of the endolymphatic duct, which arises from the labyrinth of the inner ear (connecting the utricle to the saccule). It is found in all species and controls pressure, fluid and ion levels in the endolymph. The duct extends caudally and laterally from the inner ear and the sac is located partially within the osseous labyrinth and terminates in the cranial fossa within the dura.3 The sac consists of a series of interconnected longitudinally oriented tubules or dilated crypts or cisternae, lined by a single layer of cuboidal to columnar epithelial cells, supported by a fibrovascular stroma.3,4 The endolymphatic duct and sac contain small amounts of endolymph, which can be markedly increased in the disorder, Meniere’s disease or labyrinthine hydrops, a disease of complex pathophysiology.5 Since the endolymphatic system in the middle ear is thought to influence endolymph levels in the inner ear through absorption and secretion,3 increased expression of aquaporin2 is thought to be responsible for fluid accumulation within the inner ear in some patients with Meniere’s disease.6 In geckos, dilated calcium-containing endolymphatic sacs that extend beyond the cranial vault along the cervical region appear to be an ancestral trait.7 The sacs contain radiodense aggregates of calcium carbonate, in the form of aragonite crystals,7 as seen in the radiographic images of both endolymphatic sacs of the gecko in this report. In reptiles, the sac is thought to serve as a readily available source of calcium to facilitate bone growth and egg shell formation (which is rich in calcium in geckos)7 and is colloquially called the “chalk gland or sac”.
Endolymphatic sac tumors or low grade papillary adenocarcinomas occur in humans and are one of the tumors associated with von Hippel-Lindau disease (caused by a genetic mutation in the von Hippel-Lindau gene, which is a tumor suppressor gene). The tumor can also occur sporadically, usually in patients older than those with von Hippel-Lindau disease. It is a rare tumor that behaves in a benign but locally invasive fashion. Endolymphatic tumors typically consist of papilliferous to sometimes cystic arrangements of uniform epithelial cells with low mitotic activity and an associated stroma that is frequently inflamed or hemorrhagic.4,8 Differential diagnoses include other epithelial tumors (middle ear adenoma, choroid plexus papilloma, metastatic carcinoma, thyroid adenoma [particularly the cystic variants of endolymphatic carcinoma, which can contain material that resembles colloid, that is positive with periodic-acid Schiff stain, within the cystic dilatations4]), neural or neuroendocrine tumors (paraganglioma, meningeal and ependymal tumors, chordoma) and benign lesions, such as a cholesteatoma.4,8 Endolymphatic papillary adenocarcinoma can be distinguished from these other tumors by the characteristic tubular to papilliform arrangements, local invasion and immunohistochemical staining. The tumors can be positive for cytokeratin, vimentin and neural markers, such as neurone-specific enolase and glial fibrillary acidic protein, but lack staining for S-100, calretinin and thyroid markers such as thyroid transcription factor-1 and transthyretin.4 There are two reported cases of endolymphatic tumors in dogs. The tumors were positive for cytokeratin but negative for thyroglobulin, synaptophysin, and chromogranin on immunohistochemical staining.9 The histologic findings of the two tumors in the left endolymphatic sac of this gecko were similar to that described in the human and canine cases, with the exception of the abundant calcium carbonate crystals (extracellularly and phagocytized), which is likely a consequence of the unique calcium storage function of this organ in geckos.
Various types of cancer, including those of hematopoietic, epithelial and mesenchymal origin, have been identified in geckos.10,11 To our knowledge, this case was the first description of an endolymphatic tumor in this species.2 Although the tumor did not show boney invasion, the disorganized arrangements and cellular atypia led to the histologic diagnosis of a carcinoma in situ. Although there are only low numbers of animals in published “normal” comparison data,1 there does appear to be relevant abnormalities in the hematologic and biochemical data. The anemia (presumably non-regenerative; blood smear not available to review for a regenerative response) and hypoalbuminemia can be attributed to inflammation (bone marrow suppression and a negative acute phase response, respectively). Other mechanisms for these changes cannot be ruled out. The calcium concentration is excessively high (even for an ovulating animal) per one source.12 We speculate that the hypercalcemia was due to excessive absorption of calcium from or within the neoplastic endolymphatic sac, secondary to disruption of the normal epithelial lining or prominent phagocytosis of crystals by macrophages. Other causes, such as renal disease or paraneoplastic production of a calcium-regulating hormone, are possible, although hypercalcemia has not been reported in humans with this tumor (Question 3).
References
- International Species Information System (ISIS, now called Species 360 or global information serving conservation). Physiologic data reference values – 2002. Edited by JA Teare. Values provided are for Tokay gecko.
- Sander SJ, Ossiboff RJ, Stokol T, et al. Endolymphatic Sac Carcinoma In Situ in a Tokay Gecko (Gekko gecko). Journal of Herpetological Medicine and Surgery 2015;25:82–86.
- Lo WW, Daniels DL, Chakeres DW, et al. The endolymphatic duct and sac. AJNR Am J Neuroradiol 1997;18:881–887.
- Sun Y-H, Wen W, Wu J-H, et al. Endolymphatic sac tumor: case report and review of the literature. Diagn Pathol 2012;7:36.
- Nakashima T, Pyykkö I, Arroll MA, et al. Meniere’s disease. Nat Rev Dis Primers 2016;2:16028.
- Asmar M-H, Gaboury L, Saliba I. Ménière’s Disease Pathophysiology: Endolymphatic Sac Immunohistochemical Study of Aquaporin-2, V2R Vasopressin Receptor, NKCC2, and TRPV4. Otolaryngol Head Neck Surg 2018;158:721–728
- Bauer AM. Extracranial Endolymphatic Sacs in Eurydactylodes (Reptilia: Gekkonidae), with Comments on Endolymphatic Function in Lizards. Journal of Herpetology 1989;23:172–175.
- Virk JS, Randhawa PS, Saeed SR. Endolymphatic sac tumour: case report and literature review. J Laryngol Otol 2013;127:408–410.
- Barnes KJ, Clear V, Youmans K, et al. Endolymphatic Sac Tumor in Two Dogs. Vet Pathol 2017;54:683–685.
- Garner MM, Hernandez-Divers SM, Raymond JT. Reptile neoplasia: a retrospective study of case submissions to a specialty diagnostic service. Vet Clin North Am Exot Anim Pract 2004;7:653–671.
- Hernandez-Divers SM, Garner MM. Neoplasia of reptiles with an emphasis on lizards. Vet Clin North Am Exot Anim Pract 2003;6:251–273.
- Campbell TW. Clinical pathology of reptiles. In: Reptile Medicine and Surgery, ed 2. Edited by Mader DR. Elsevier, St. Louis, MO. 2006; pp 453-470.
Authored by: T. Stokol (with permission from co-authors of reference #2).