Physiology
Bile acids are a group of amphipathic steroids synthesized by hepatocytes from cholesterol and excreted into bile. They function to emulsify fat in intestine and facilitate nutrient absorption and are highly conserved via enterohepatic circulation as outlined below. This emulsification is possible because of their amphipathic nature and tendency to form micelles.
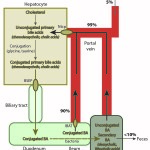
To produce bile acids, cholesterol is first degraded to a “primary” bile acid (mostly cholic acid or chenodeoxycholic acid) via hydroxylase enzymes. The primary bile acids are then conjugated to glycine, taurine (particularly in cats), glucuronic acid and sulfates within the hepatocyte and excreted into bile canaliculi via the bile salt export pump (BSEP) and multidrug resistance-associated protein-2 (also transports conjugated bilirubin). The excretion of bile acids into the biliary canaliculi sets up an osmotic gradient, which draws water into bile and drives bile salt-dependent bile flow. Once in the intestine, conjugated bile acids are reabsorbed by active transport receptor-mediated mechanisms in the ileum, which is greater than 90% efficient. The receptor is called the intestinal bile acid transporter (IBAT) and is structurally related to the uptake transporter on the canalicular surface of the hepatocyte (Ntcp) that extracts bile acids from blood (and is also sodium-dependent). In dogs, the IBAT protein is primarily expressed in the ileum, cecum and colon. The receptor is downregulated in dogs with chronic intestinal inflammation, leading to increased loss of primary bile acids in feces (Giaretta et al 2018). Bile acids that are not absorbed (10% or less) can be deconjugated by intestinal bacteria (particularly if they pass into the colon, but also in the ileum) and are then passively absorbed in the colon. Colonic absorption of these unconjugated bile acids will be increased in malabsorption due to ileal disease or resection when more bile acids are presented to the colon. Bile acids can also be dehydroxylated by enteric bacteria to produce “secondary” bile acids (deoxycholic acid and lithocholic acid), which are also absorbed in the colon or excreted in the feces. After absorption, the unconjugated and conjugated bile acids enter the portal blood, where >95% are extracted by the hepatocyte via a sodium-dependent taurocholate cotransporter (Ntcp – this takes up conjugated and some unconjugated bile acids) and the family of organic anion transporting polypeptides (OATP – this take up unconjugated bile acids, and likely unconjugated and conjugated bilirubin). Thus, very small amounts of bile acids (generally <15 μmol/L) are seen in fasting blood samples of dogs and cats due to the efficiency of ileal resorption and hepatic extraction of the recycled bile acids. Naturally, gall bladder contraction in these species will result in an extra load of bile acids being excreted into the intestine and reabsorbed, so higher concentrations are expected after gall bladder contraction (which we mimic in the clinic by providing food and then measuring a post-prandial bile acid concentration – the goal of which is to “challenge” the liver to see if it can handle this extra load). Note that both the excretion of bile acids into the canaliculi and extraction of bile acids from the portal blood involve energy-dependent pumps which can be disrupted in a variety of conditions including cholestasis from obstruction or inflammation (cytokine-mediated).
There are some confusing terms associated with bile acids (which we have used here as a generic term).
- Bile acid actually refers to the unconjugated forms (produced in the liver from cholesterol and in the intestine, the latter by deconjugating action of enteric bacteria)
- Bile salt more accurately refers to the conjugated form, which is produced in the hepatocyte. Conjugation increases their water solubility, preventing passive re-absorption once secreted into the small intestine. As a result, the concentration of bile acids in the small intestine can stay high enough to spontaneously form micelles (called critical micellar concentration) and solubilize lipids. Bile salts are also more efficient at emulsifying fats than bile acids because at intestinal pH, they are more electrically charged.
- Primary bile acids are formed by synthesis in the liver and consist of chenodeoxycholic acid and cholic acid (which can be conjugated or unconjugated).
- Secondary bile acids are those made by bacteria in the gut, specifically by dehydroxylating primary bile acids. As a result, lithocholic acid (dehydroxylated chenodeoxycholic acid) and deoxycholic acid (dehydroxylated cholic acid) are secondary bile acids. These bile acids are poorly water soluble and toxic to cells.
Methods
The following method is used by Cornell University to measure bile acids.
Reaction type
Kinetic/enzymatic reactions
Procedure (5th generation)
The enzyme, 3α-hydroxysteroid dehydrogenase (3α-HSD) catalyzes the oxidation of the bile acids and simultaneously converts thio-NAD to thio-NADH. The enzyme can also catalyze the reverse direction and reform thio-NAD, however the enzyme cycling continues in the presence of excess NADH. The rate of formation of thio-NADH is measured by an absorbance change at 405 nm after sample blanking and is equivalent to the bile acid concentrations (i.e. more cycling will occur if bile acid concentrations are higher).
Units of measurement
At Cornell University, the results are reported in μmol/L (SI units).
The conversion from conventional (mg/mL) to SI (mmol/L) units is as follows:
Total bile acid: mg/mL x 2.547 = mmol/L
Sample considerations
Sample type
Serum, plasma
Timing
In dogs and cats, both random (not related to eating or fasting), fasting (preferably overnight or collected just before the routine meal) or post-prandial (2 hours after eating a standard meal) bile acid concentrations can be measured, although a bile acid panel, consisting of fasting and post-prandial samples is preferred due to its increased sensitivity to hepatic dysfunction. This is done because it is a “challenge” test – gall bladder contraction initiated by feeding creates a bolus of bile acids for the liver to extract after intestinal absorption. This is not done in grazing animals (also horses lack a gall bladder). Both blood samples should be collected into red-top vacutainers (serum is preferred for bile acid measurement) and serum should be separated promptly from cells.
Anticoagulant
Heparin is acceptable.
Stability
If not processed on the same day, refrigeration at 2 – 8°C or freezing at -20°C is required.
Interferences
- Lipemia, hemolysis: Lipemia (falsely increases) and hemolysis (falsely decreases) do interfere with bile acid measurement through optical interference with older reagents and methods (Solter et al 1992). Per the manufacturer application sheet, a triglyceride concentration up to 750 mg/dL and hemoglobin concentration up to 500 mg/dL should not substantially interfere with the bile acid concentration. This statement has not been formally tested in animal samples.
- Icterus: High bilirubin per se does not interfere with test measurement. It is more that testing for bile acids should not be performed in an animal with high direct bilirubin concentrations or icterus due to cholestasis or that has any biochemical evidence of cholestasis (high total and direct bilirubin, bilirubinuria), since in these cases, the test cannot be relied upon to give any additional information about liver function or vascular flow defects (e.g. shunting).
Test interpretation
Bile acid measurement provides useful information about the portal venous circulation and hepatic function. They are produced in the liver from cholesterol and are stored in the gall bladder. Gall bladder contraction with feeding releases bile acids into the intestine. Bile acids undergo enterohepatic circulation, i.e. they are absorbed in the intestine and taken up by hepatocytes for re-excretion into bile. Measurement of bile acid concentrations is, therefore, a good indicator of hepatobiliary function, but is not specific for the type of underlying liver disease and diseases that secondarily affect the liver (e.g. metabolic diseases like hyperadrenocorticism) can also increase bile acid concentrations. They are also used as a marker of abnormal portal blood flow.
Increased concentration
Bile acid concentrations will be increased under the following situations:
- Hepatocellular dysfunction: Inability of the hepatocyte to produce or extract bile acids from the portal circulation.
- Abnormal portal blood flow: Portosystemic shunts or microvascular dysplasia (now called portal vein hypoplasia) will cause portal blood to “bypass” the liver, not allowing hepatocytes to efficiently extract the bile acids that would be normally presented to them. Abnormal blood flow also results in liver dysfunction (loss of trophic substances).
- Cholestasis: Any interference with the transporters that deliver bile from the hepatocyte to the biliary canalicular system will result in an increase in bile acid concentrations (some of these transporters, such as MRP2, are shared with conjugated bilirubin). This will occur in structural and “functional” cholestasis. This is not only because bile salts are “retained” in the hepatocyte or reflux via cholestasis-induced alterations in the hepatocyte cytoskeleton causing cellular retraction and paracellular permeability, but under conditions of cholestasis, certain sinusoidal transporters (MRP3, 4, and the organic solute transporters, OSTα and OSTβ) are also induced or stimulated to pump the bile salts back into the circulation. Thus, there is no point in measuring bile acids in animals that are known to be cholestastic (increased direct bilirubin etc), because the cholestasis will mask our ability to detect hepatic dysfunction or abnormal blood flow. Through their emulsifying action on membranes, bile acids are toxic to cells and their build up within hepatocytes in cholestatic conditions can result in hepatocellular injury and altered signaling, leading to fibrosis and aberrant growth, including hepatocellular carcinoma formation (at least in rodent models). Hepatocytes have developed techniques to reduce bile acid toxicity in cholestatic conditions, including reversal of transporters to pump bile acids back into blood, as indicated above, and downregulation of bile acid production, through binding to nuclear receptors, such as the Farsenoid X receptor (Li and Apte 2015).
Note, that high bile acid concentrations are not specific for underlying liver dysfunction or abnormal portal blood flow. Increases can be seen in animals without clinical or other laboratory evidence of liver disease (e.g. dental disease in dogs [Center SA, personal communication]).
Variables affecting test interpretation
Several variables can affect the interpretation of bile acid testing:
- Interferences: Hemolysis and lipemia both affect test results (see above).
- Lower concentrations: Prolonged fasting, intestinal malabsorption, rapid gastrointestinal transit, delayed gastric emptying or ineffective gall bladder contraction may lower bile acid concentrations and decrease the sensitivity of bile acid testing for hepatobiliary disease.
- Increase fasting concentrations in dogs and cats: Gall bladder contraction during the fast.
Species-specific comments
- Dogs and cats: Measurement of both fasting and two hour post-prandial bile acids can be useful. Feeding stimulates gall bladder contraction which releases bile acids into the intestine and portal circulation (after intestinal absorption). This increases the load of bile acids that must be extracted from blood by the liver and increases the sensitivity of the procedure to hepatobiliary or vascular defects. Young dogs of breeds predisposed to congenital portosystemic shunts should be tested when greater than 16 weeks of age, because bile acid concentrations may be falsely lower in animals younger than this (Dr. Center, personal communication). For stimulation testing, animals should be fed at their routine meal times (e.g. morning) and should be given their regular meal (amount and type). Note, that when interpreting the results, the higher of the two concentrations should be taken versus relying on the postprandial to always exceed fasting concentrations (see below).
- Bile acid concentrations >25-30 μmol/L in dogs and > 25 μmol/L in cats are suggestive of hepatobiliary disease, i.e. decreased functional mass, alterations in portal circulation (or cholestasis, but this test is not recommended in cholestatic animals). These guidelines are valid for pre-prandial (fasting), post-prandial and random (unrelated to eating) samples.
- Based on studies done by Dr. Center at Cornell University, dogs with bile acid concentrations < 15 μmol/L do not have evidence of hepatic pathology on biopsy, whereas dogs with values > 25 μmol/L usually have hepatic pathology. Dogs with bile acid values between 15-25 μmol/L are in an equivocal zone (i.e. may or may not have hepatic pathology).
- Postprandial > preprandial concentrations: Most animals have higher post-prandial than fasting bile acid concentrations, however some animals (up to 20% of dogs) may have higher fasting than post-prandial bile acid concentrations, due to a recent meal, gall bladder contraction during fasting, or delayed gastric emptying. In this scenario, if both results are < 25 μmol/L (especially < 15 μmol/L), hepatobiliary disease is unlikely.
- Most animals with congenital or acquired portosystemic shunting have markedly increased post-prandial bile acids concentrations. This is likely due to a combination of portal blood bypassing the liver and decreased functional mass, a frequent concurrent finding in affected animals.
- Horses: Horses lack gall bladders and only random bile acid concentrations are measured in these species.
- Slightly increased concentration (up to approximately 20 μmol/L) can result from decreased feed intake for a period of several days or longer.
- In one study, higher bile acid concentrations were associated with decreased survival and inflammatory or portal fibrotic pathologic lesions in the liver (Dunkel et al 2015). Other studies have found similar associations with survival (McGorum et al 1999, Durham et al 2003).
- In horses with experimentally induced hepatitis from equine parvovirus, increases in bile acid concentrations were seen and usually paralleled changes seen in GGT activity, often being seen after the peak of increase in SDH and GLDH, hepatocellular injury enzyme, activities. Increased in bile acid concentration were only seen in horses with GLDH activity >120 U/L. The horse with the highest increase in bile acid concentrations also had evidence of cholestasis, with a mild increase in direct bilirubin concentration. These results imply that the increase in bile acid concentrations is due to biliary issues, likely cholestasis. In contrast to GGT activity, bile acid concentrations were only up transiently, suggesting a reversible cholestatic syndrome (Tomlinson et al 2020).
- Note that young foals under 2 weeks of age can have higher bile acid concentrations than adults. Bile acid concentrations approximate adults by about 6 weeks of age in foals (Barton and LeRoy 2007).
- Ruminants: Ruminants have extremely variable serum bile acids concentration in health, rendering the test insensitive for the detection of liver dysfunction (shunts are very rare in ruminants).
- Camelids: Since camelids are constant grazers, only random bile acid measurements are performed.
- Bile acid concentrations increase with hepatic lipidosis in llamas. This may be a consequence of cholestasis or may reflect hepatocellular dysfunction in non-cholestatic animals (Tornquist et al 1999).
- Bile acid concentrations are greater in llamas < 1 year of age versus those older than 1 year of age (Andreasen et al 1998).
- Fasting does not appear to affect bile acid concentrations (Andreasen et al 1998).
