Interpretation
Extramedullary plasmacytoma with amyloid
Explanation
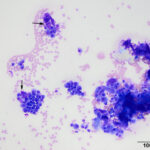
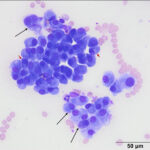
The smears were highly cellular and composed of a round (discrete) cell population (Question 1) in a mildly bloody background (Figures 1-3). The cells were round to oval with a moderate nuclear to cytoplasmic ratio. Their nuclei were round to oval to rarely indented, eccentric to paracentrically placed with coarsely stippled to clumped chromatin. They had a moderate amount of medium to deep blue cytoplasm, occasionally containing variable numbers of small to rarely large, medium light pink to purple granules (intracellular amyloid, presumptive). A few of the cells had a distinct perinuclear clear zone (Figure 2). They displayed mild to moderate anisocytosis and anisokaryosis with frequent binucleation and occasional multinucleation. A few of the cells had large pleomorphic nuclei with finer chromatin. The cytologic diagnosis was an extramedullary plasmacytoma (Question 2). The cells were associated with and elaborating an abundant amount of smooth globular to chunky medium to dark purple extracellular material, compatible with amyloid (Figures 1, 3, 4) (Question 3).
Discussion
Canine extramedullary plasmacytoma (EMP) can be cutaneous or noncutaneous. They are within the group of plasma cell neoplasms that includes multiple myeloma (disseminated or multisystemic plasma cell tumor) and solitary plasmacytoma or myeloma of bone.1 In dogs, the extramedullary tumors are most commonly found in the skin, mainly on the head (face, lip, and ear canals) and paws, and mucocutaneous junctions. Internal tumors in visceral organs, such as the gastrointestinal tract, also occur.2–4 There appears to be an over-representation of cutaneous EMP in certain dog breeds, including spaniels (Cocker and Springer), terriers (West highland white and Yorkshire), and boxers.2,5,6 EMPs occur mainly in middle aged to older dogs and there is no consistently reported sex predilection.1,2,7 Most of the tumors are solitary, but there have been reports of multiple tumors without an association with multiple myeloma.8 These tumors are considered benign with less than 1% of cutaneous EMP being associated with multiple myeloma and there is rare evidence of metastasis to other organs.4,5,8,9 One report documented progression of a cutaneous EMP to a plasma cell leukemia in a 5 year old male Bernese mountain dog.10 Grossly, these tumors have a nonspecific appearance. They can be raised, firm, nodular to occasionally multilobulated masses, usually measuring less than 2 cm in diameter, that are alopecic or lightly haired and rarely ulcerated.5,9 Their gross appearance mimics that of a histiocytoma.
On cytologic smears, plasmacytomas fit into the category of discrete or round cell tumors. When well-differentiated, they are readily distinguished from other round cell tumors, primarily histiocytic tumors such as a histiocytoma, by their eccentric nuclei with clumped chromatin, dark blue cytoplasm and perinuclear clear zones. Their nuclei tend to be rounder and less indented than histiocytomas, which is the main differential diagnosis for a cutaneous plasma cell tumor. Bi- or multinucleated cells displaying moderate to marked intracellular anisokaryosis and round nuclei is also a characteristic feature of plasmacytomas versus histiocytomas. Binucleated and multinucleated cells can be seen in the latter tumors but they usually have larger and more irregularly shaped nuclei with lightly stippled chromatin. Plasmacytomas also lack the proteinaceous background that is typically, but not invariably, seen in histiocytomas. In poorly cellular or smudged samples (or Diff-quik®-stained smears), these distinguishing features may be difficult to discern. Extracellular amyloid would also be indicative of a plasma cell tumor, but can be difficult to differentiate from necrotic cells or necrotic cellular debris. Amyloid would lack fading nuclei that is seen in necrotic cells and has a different color than necrotic cells or debris, the latter being “muddy” red-purple on Wright’s-stained smears (color changes are hard to appreciate on Diff-quik®).
On histological sections of formalin-fixed paraffin-embedded tissue, cutaneous and mucocutaneous EMPs are densely cellular and comprised of round to polygonal cells that are arranged in sheets to tight nests.9 The cells can display various morphologic features that have been divided into 5-6 subtypes.3,7 These histological subtypes consist of a hyaline type, mature type, cleaved type, asynchronous type, and polymorphous-blastic type3 with a 6th subtype of monomorphous blastic type found in mucocutaneous EMP lesions.7 The hyaline type is characterized by light pink cytoplasm with eccentrically placed sickled-shaped nuclei whereas the mature type has cells that are more consistent with a mature plasma cell (clumped chromatin, eccentric nucleus, and amphiphilic cytoplasm with a perinuclear clear zone).3 The cleaved type is the most commonly seen type of cutaneous EMP and has a characteristic indented to cleaved nucleus with a perinuclear clear zone.3 This type also has displays moderate anisocytosis and the cells are separated into indistinct lobular-like arrangements by fibrous septa.3 The asynchronous type is characterized by nuclear to cytoplasmic asynchrony.3 The cells have an eosinophilic, vacuolar cytoplasm containing a perinuclear clear zone with a more immature, blastic nucleus and a central nucleolus.3 The last subtype within the cutaneous EMP is the polymorphous-blastic type.3 These cells are the most pleomorphic and often lack a perinuclear clear zone.3 Multinucleated giant cells are frequent with higher numbers of nuclei within individual cells as the tumor becomes more pleomorphic.3 The monomorphous type of mucocutaneous EMPs is characterized a monomorphic population of tumor cells with large euchromatic nuclei, a centrally placed small nucleoli, and rare multinucleated giant cells.7 Both studies that defined these 5-6 types of EMPs found that categorization can help in the diagnosis of EMPs but could not be used for grading or predicting biological behavior and prognosis.3,7
An interesting cytological in this case, which is not seen in many aspirates of these lesions, was the abundant amount of extracellular matrix consistent with amyloid. Low numbers of cutaneous EMPs, about 10-12.5%, can have localized deposition of amyloid.7,9 Amyloid is a fibrillar protein composed of insoluble β-pleated sheets and appears as a smooth, eosinophilic matrix with hematoxylin and eosin stain on histologic samples or as a smooth, globular to chunky, medium to deep purple matrix with modified Wright’s stain.6 There are two forms of amyloid, amyloid A (AA) and amyloid L (AL).5,6 Amyloid A is also known as secondary (reactive) amyloid because it usually deposits in response to chronic inflammation and is comprised of the amino-terminal portion of the acute phase protein, serum amyloid A.5,6 Whereas amyloid L, also known as primary amyloid, is formed from amyloidogenic precursors, such as the amino terminal fragments and complete immunoglobulin light chains that are produced by lymphoplasmacytic tumors.5,6 AL production within canine cutaneous EMPs can been confirmed by positive Congo red staining (green stain) that is resistant to potassium permanganate and positive immunohistochemical staining with antibodies against human or equine AL amyloid.5,6 Use of these staining techniques can be beneficial when the diagnosis of an EMP is difficult by low numbers of tumor cells and or extensive amyloid deposition.5 As with the subtyping of these tumors, the presence or absences of amyloid deposition cannot help predict biological behavior or prognosis.7
Follow-up
The patient was referred to a specialty practice for surgical removal of the interdigital mass. The mass was successfully excised without affecting the digits and the defect was closed by performing a podoplasty. The tumor was submitted for histopathologic examination, which confirmed the cytologic diagnosis of a cutaneous extramedullary plasmacytoma. A subtype was not provided. On last follow-up, the patient was doing well with no evidence of tumor recurrence.
References
- Mikiewicz M, Otrocka-Domagała I, Paździor-Czapula K, Gesek M. Morphology and immunoreactivity of canine and feline extramedullary plasmacytomas. Pol J Vet Sci. 2016;19(2):345-352.
- Lucke VM. Primary cutaneous plasmacytomas in the dog and cat. J Small Anim Pract. 1987;28(1):49-55.
- Platz SJ, Breuer W, Pfleghaar S, Minkus G, Hermanns W. Prognostic Value of Histopathological Grading in Canine Extramedullary Plasmacytomas. Vet Pathol. 1999;36(1):23-27.
- Trevor PB, Saunders GK, Waldron DR, Leib MS. Metastatic extramedullary plasmacytoma of the colon and rectum in a dog. J Am Vet Med Assoc. August 1;203(3):406-409.
- Rowland PH, Valentine BA, Stebbins KE, Smith CA. Cutaneous Plasmacytomas with Amyloid in Six Dogs. Vet Pathol. 1991;28(2):125-130.
- Platz SJ, Breuer W, Geisel O, Linke RP, Hermanns W. Identification of k Light Chain Amyloid in Eight Canine and Two Feline Extramedullary Plasmacytomas. J Comp Path. 1997;116:45-54.
- Cangul IT, Wijnen M, van Garderen E, van den Ingh TSGAM. Clinico-pathological Aspects of Canine Cutaneous and Mucocutaneous Plasmacytomas. J Vet Med Ser A. 2002;49(6):307-312.
- Boostrom BO, Moore AS, DeRegis CJ, Robat C, Freeman K, Thamm DH. Canine Cutaneous Plasmacytosis: 21 Cases (2005–2015). J Vet Intern Med. 2017;31(4):1074-1080.
- Gross TL, Ihrke PJ, Walder EJ, Affolter VK: Cutaneous amyloidosis and Cutaneous plasmacytoma In: Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis. 2nd ed. Oxford, UK: Blackwell Science; 2005. p. 383-386 and 866-872.
- Rout ED, Shank AMM, Waite AHK, Siegel A, Avery AC, Avery PR. Progression of cutaneous plasmacytoma to plasma cell leukemia in a dog. Vet Clin Pathol. 2017;46(1):77-84.
Authored by: S. Chu (resident) and edited by T. Stokol