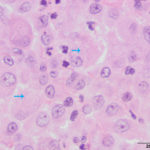
Interpretation
Septic pyogranulomatous inflammation.
Explanation
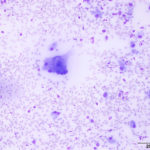
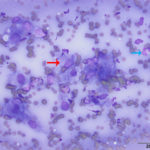
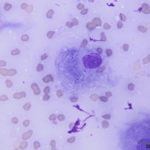
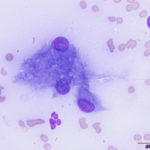
The aspirate was of moderate cellularity, consisting of epithelioid macrophages (Question 1, red arrow) and fewer degenerate neutrophils (Question 1, blue arrow). The cells were present individually and in small aggregates on a proteinaceous background with moderate amounts of blood. There were occasional binucleated and multinucleated variants of macrophages. Often, the macrophages contained numerous filamentous bacteria, several of which had an oval to round, basophilic expanded end. The bacteria were also sometimes poorly-staining and appeared as negative images. The filamentous appearance of the bacteria and the pattern of inflammation were suggestive of infection with bacteria such Actinomyces or Nocardia spp. (Question 2). Aerobic and anaerobic bacterial culture with notification to the laboratory of the suspicion of actinomycosis or nocardiosis was recommended (Question 3).
Additional Diagnostic Testing
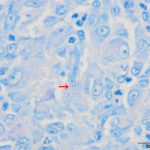
The cat was taken to surgery for removal of the mass, which was submitted for histopathologic examination. Additionally, swabs of the mass were submitted for aerobic and anaerobic bacterial cultures. Histopathologic evaluation of the mass revealed focal pyogranulomatous panniculitis with small numbers of intracellular filamentous bacteria suggestive of infection by Nocardia or Actinomyces spp. (Figure 5). Additional histochemical stains to highlight and further characterize the bacteria were done. A Ziehl-Neelsen stain showed no organisms and a Fite-Faraco stain variably highlighted the bacteria within macrophages (Figure 6). A Grocott’s methenamine silver stain (GMS, Figure 7) strongly highlighted the bacteria. Rare bacteria were seen on a Gram-stained smear (not shown) and results were inconclusive as to the species of bacteria.
The aerobic bacterial culture yielded many Gram-positive branching rods, which on further speciation via DNA sequencing were consistent with Nocardia paucivorans. No growth was observed on the anaerobic bacterial culture.
Follow-up
The cat recovered from surgery without complications. The doxycycline therapy was discontinued and antimicrobial treatment with trimethoprim-sulfonamide (TMS) was started instead. Eight days post-surgery the incision was healing well.
Discussion
Nocardia spp. are Gram-positive aerobic bacteria that are ubiquitous soil saprophytes and also present in fresh and salt water, dust, decaying plant material, and feces. Unlike Actinomyces spp. they are not part of the normal flora of mammals, but they can be mechanically carried on the claws or skin.1,2 Nocardiosis is rare in dogs and cats, but may result from inadvertent direct inoculation into the skin via puncture or penetrating wounds (such as scratch and bite wounds) or via inhalation of bacterial particles into the lungs.1,2 Transmission between animals has not been reported.1 There is an apparent increased incidence of infection associated with immunosuppression and male cats are over-represented. The latter is thought to reflect the greater likelihood of male cats to roam and fight.1–3 A puncture wound was considered the most likely source of infection in this cat, since the cat had been seen fighting with a rat, which resulted in an abrasion over the left eye.
Nocardiosis is classified into three main clinical presentations: 1) Pulmonary, 2) Systemic, and 3) Cutaneous and subcutaneous. Of these three presentations, cutaneous and subcutaneous nocardiosis are the most common in cats. These forms typically cause localized skin nodules, cellulitis, and abscesses that progress to fistulous tracts with a serosanguinous discharge. Although uncommon compared to Actinomyces spp., yellow granules (sulfur granules) in the exudate may be noted. The skin lesions are usually formed in the extremities, inguinal area, flank, bridge of the nose, and as seen in this cat, the neck.1,3
Cytologic and histopathologic evaluation of the lesions may reveal neutrophilic to pyogranulomatous inflammation with activated macrophages (including giant multinucleated and epitheliod forms), tissue necrosis, and filamentous bacteria within macrophages. Extracellular aggregates of bacteria (tissue granules) may also be present. The bacteria are irregularly staining and form thin (<1 um wide) branching filaments that may break into pleomorphic rod-shaped or coccoid elements.1,2,4 The activation of macrophages into giant multinucleated and epithelioid forms results from the ability of Nocardia organisms to inhibit phagosome-lysosome fusion, phagosomal acidification, and oxygen-dependent bactericidal mechanisms.1
Infectious agents that may cause similar skin lesions and inflammatory patterns in small animals, include Actinomyces spp., Mycobacterium spp., Rhodoccocus spp., Corynebacterium spp., Dermatophilus congolesis, and saprophytic fungi.2 Of these, Actinomyces organisms are cytomorphologically the most similar to Nocardia, both consisting of filamentous, branching and beading bacteria. The specific identification of filamentous bacterial infections is critical as the antibiotic treatment for Actinomyces and Nocardia spp. infections differ. Specific identification can be achieved via bacterial culture, histochemical, and molecular methods.
Nocardia organisms grow aerobically in simple media, however longer than standard incubation times (2-4 weeks) or modification of culture conditions to enhance their growth may be needed for isolation. Therefore, a laboratory should be alerted if nocardiosis is suspected. Actinomyces spp. are facultative or obligate anaerobes and are primarily cultured anaerobically. Both Nocardia and Actinomyces spp. are Gram-positive bacteria and are highlighted by GMS. Unlike Nocardia, which is weakly acid-fast, Actinomyces is not acid-fast, however. The Fite-Faraco stain is a modified acid-fast stain and compared to the Ziehl-Neelsen method, is more sensitive to nocardiosis.1,2 In this case, the histochemical staining showed the bacteria were weakly acid-fast with Fite-Faraco, a staining pattern most consistent with Nocardia spp. Nocardiosis was further supported by the results of the aerobic bacterial culture that showed many aerobic Gram-positive branching rods. Given the results of the Gram-stain performed during the bacterial culture, the inconclusive result of the histochemical Gram-stain was thought to be a technical issue versus an inherent characteristic of the bacteria.
The clinical course of cutaneous nocardiosis in cats appears to be highly variable, presenting as a chronic, indolent localized disease to a fulminating disseminated infection with high mortality.1 This high mortality is however, partly attributed to delayed or inadequate therapy, whereas early and appropriate therapy appear to result in a prompt response.1,2 Ideally, treatment of Nocardia infection should involve appropriate antimicrobial therapy with one or a combination of agents combined with surgical drainage or removal of the lesions.1,4 Most Nocardia species are susceptible to sulfonamides, and sulfonamides and TMS combinations are the main antimicrobial drugs used for treating nocardiosis. Antimicrobial selection may best be guided by susceptibility studies.
The antimicrobial susceptibility varies between Nocardial species and the sensitivity of several pathogenic species to various drugs in vitro has been described.1,2 If determination of antimicrobial susceptibility is not done, determining the infecting species may help in the effective antimicrobial therapy and may additionally guide future appropriate therapy. Molecular methods such as DNA sequencing via the 16S rRNA gene PCR-based method, provide fast and reliable means of speciation.1,5 In this case, determination of the infecting Nocardial species by DNA sequencing was consistent with Nocardia paucivorans, a species not previously reported in cats.
Antimicrobial susceptibility testing was not done in this case. However, given that most species of Nocardia and isolates from human cases of nocardiosis caused by N. paucivorans were susceptible to TMS,6,7 initiating therapy with this antimicrobial drug was appropriate in this case. In people with cutaneous nocardiosis, 1-3 months of antimicrobial treatment are recommended. Similar antimicrobial therapies have been used in small animals with variable success.1,2 Recurrence of infection may occur and high doses of TMS given for long periods to dogs and cats may result in myelosuppression. Unfortunately, the cat in this case was lost to follow-up and its long-term response to TMS antimicrobial therapy remains unknown.
Although rare, infection by Nocardia spp. should be considered as a differential diagnosis for cutaneous or subcutaneous abscess-like lesions, with a neutrophilic or pyogranulomatous pattern of inflammation, particularly if beaded filamentous rods are noted in aspirates. Additionally, specific and correct identification between the filamentous bacteria, Nocardia and Actinomyces spp. and speciation of Nocardia organisms, are critical for the implementation of appropriate antimicrobial therapy.
References
- Sykes JE. Actinomycosis and Nocardiosis. In: Infectious Diseases of the Dog and Cat. Fourth Edi. Elsevier; 2015. p. 484–94.
- Malik R, Krockenberger M, O’Brien C, White J, Foster D, Tisdall P, et al. Nocardia infections in cats: a retrospective multi-insitutional study of 17 cases. Aust Vet J. 2006;84(7):235–45.
- Hiromi H, Yasuyuki E, Maiko S, Asuka S, Yasuyuki M. Cutaneous Nocardiosis in a Cat. J Vet Med Sci. 2009;71(6):785–7.
- Barfield D, Rich S, Pegrum S, Malik R. What is your diagnosis? Non-healing wound on the paw of a cat. J Small Anim Pract. 2007;48:353–4.
- Rodriguez-Nava V, Couble A, Devulder G, Flandrois J-P, Boiron P, Laurent F. Use of PCR-restriction enzyme pattern analysis and sequencing database for hsp65 gene-based identification of Nocardia species. J Clin Microbiol. 2006 Feb;44(2):536–46.
- Eisenblätter M, Disko U, Stoltenburg-Didinger G, Scherübl H, Schaal KP, Roth A, et al. Isolation of <em>Nocardia paucivorans</em> from the Cerebrospinal Fluid of a Patient with Relapse of Cerebral Nocardiosis. J Clin Microbiol [Internet]. 2002 Sep 1;40(9):3532 LP-3534. Available from: http://jcm.asm.org/content/40/9/3532.abstract
- Monticelli J, Luzzati R, Maurel C, Rosin C, Valentinotti R, Farina C. Brain Abscesses Caused by Nocardia paucivorans in a Multiple Myeloma Patient Treated with Lenalidomide and Dexamethasone: a Case Report and Review of Literature. Vol. 7, Mediterranean Journal of Hematology and Infectious Diseases. 2015.
Authored by: D. Hernandez and edited by T. Stokol
The authors thank Drs. Robin Allison and Mason Jaeger for their assistance in interpreting the microscopic results.